
The electronic structure of $S{O_2}$ molecules is best represented as a resonance hybrid of how many resonating structures?
A.2
B.3
C.4
D.5
Answer
566.7k+ views
Hint: Resonance structure are different forms of a specific molecule in which there is a difference in the distribution of electrons within the same molecule but there is no change in their connectivity. The electronic structure of $S{O_2}$ molecules is best represented as a resonance hybrid of two resonating structures.
Complete step by step answer:
Sulphur dioxide is the entity of a bond between sulphur and oxygen atoms. Its formula is written as. $S{O_2}$ The molecular shape of $S{O_2}$ is the same as the molecular geometry of carbon dioxide $S{O_2}$ .
Sulphur dioxide has two resonance structures which contribute equally to the overall hybrid structure of the molecule. A third Lewis structure can also be drawn for sulphur dioxide which is more stable.
The total number of valence electrons is 18, out of which 6 is from sulphur and 6 from each of the two oxygen atoms. $S{O_2}$ molecules have two different types of pi bonds $(p\pi - p\pi )$ and $(p\pi - d\pi )$ . But still both the \[S - O\] bonds are identical. The structure is formed by completing the octet with the most electronegative element Oxygen (O). The negative charge is placed on the Oxygen whereas the positive charge is placed on sulphur, the less electronegative of the two atoms.
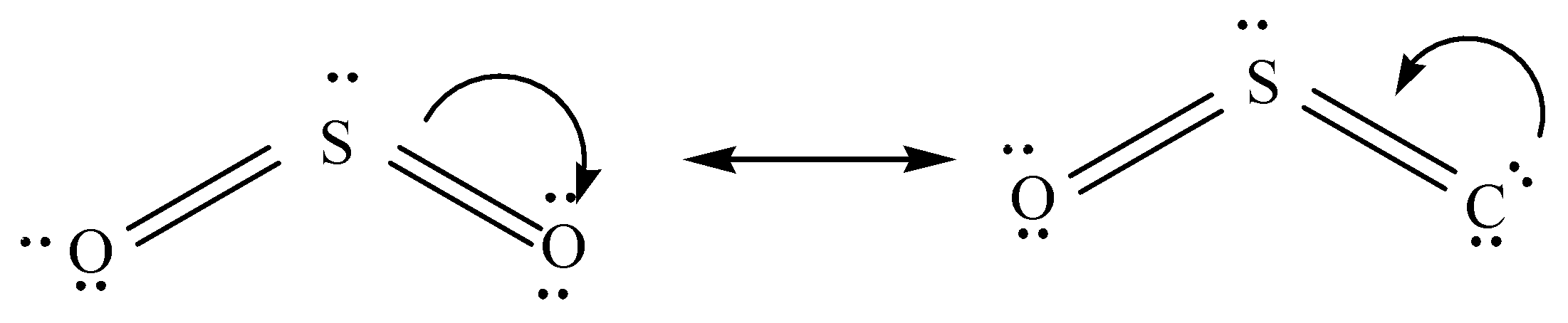
On two Oxygen atoms, the negative charge will split.
The two resonance structures are equivalent and will contribute equally to the hybrid structure.
Therefore, the correct answer is option (A).
Note: The electron geometry of $S{O_2}$ is formed in the shape of a trigonal planar. The three pairs of bonding electrons are at an angle of 120-degree in the plane. As one pair remains alone, two double pairs are bonded and thus the shape of $S{O_2}$ is a bent shape.
Complete step by step answer:
Sulphur dioxide is the entity of a bond between sulphur and oxygen atoms. Its formula is written as. $S{O_2}$ The molecular shape of $S{O_2}$ is the same as the molecular geometry of carbon dioxide $S{O_2}$ .
Sulphur dioxide has two resonance structures which contribute equally to the overall hybrid structure of the molecule. A third Lewis structure can also be drawn for sulphur dioxide which is more stable.
The total number of valence electrons is 18, out of which 6 is from sulphur and 6 from each of the two oxygen atoms. $S{O_2}$ molecules have two different types of pi bonds $(p\pi - p\pi )$ and $(p\pi - d\pi )$ . But still both the \[S - O\] bonds are identical. The structure is formed by completing the octet with the most electronegative element Oxygen (O). The negative charge is placed on the Oxygen whereas the positive charge is placed on sulphur, the less electronegative of the two atoms.

On two Oxygen atoms, the negative charge will split.
The two resonance structures are equivalent and will contribute equally to the hybrid structure.
Therefore, the correct answer is option (A).
Note: The electron geometry of $S{O_2}$ is formed in the shape of a trigonal planar. The three pairs of bonding electrons are at an angle of 120-degree in the plane. As one pair remains alone, two double pairs are bonded and thus the shape of $S{O_2}$ is a bent shape.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




