
The electronic configuration of \[He\] in one of its excited state is as follows:
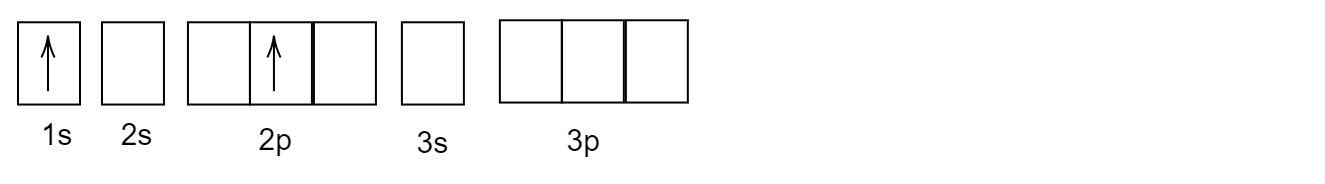
What will be the incorrect configuration(s) of the next excited state of this?
A)
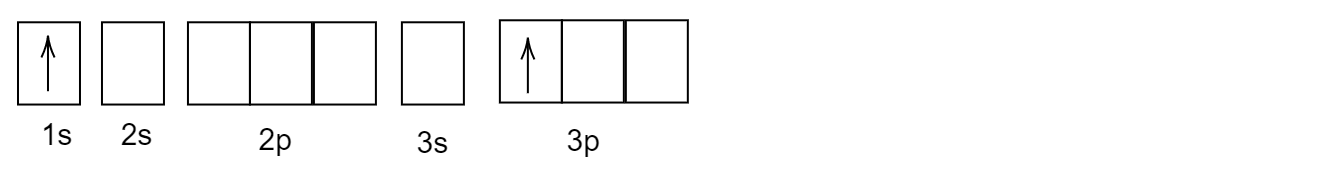
B)
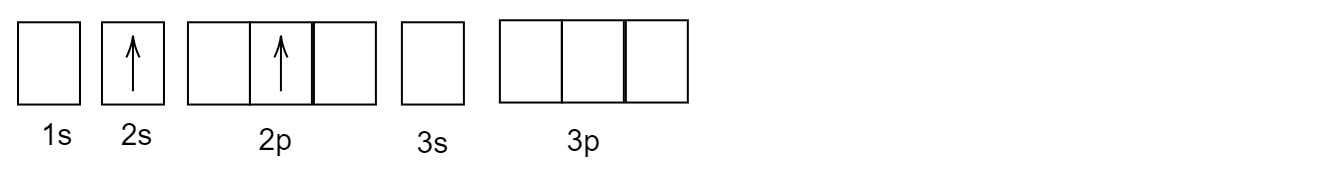
C)
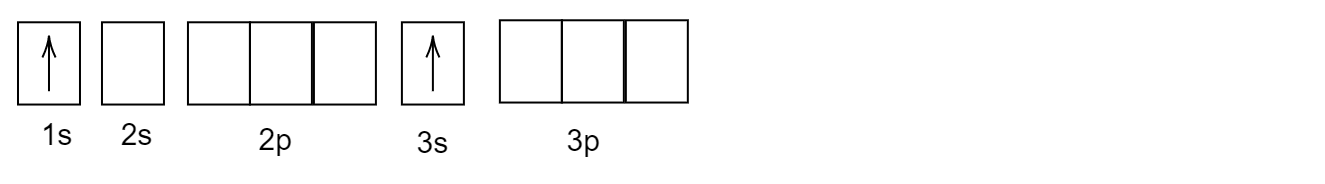
D)
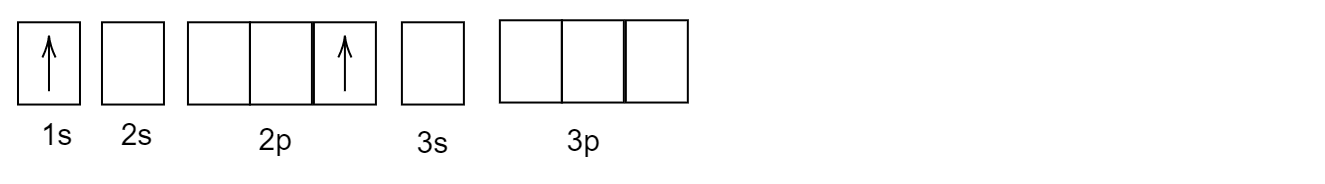





Answer
497.1k+ views
Hint: First, we need to understand the excited state configuration. It is a higher energy state than the ground state. An energy is required to create the excited state. An excited state means that the valence electrons moved from its ground state (lowest energy state to some higher energy state.
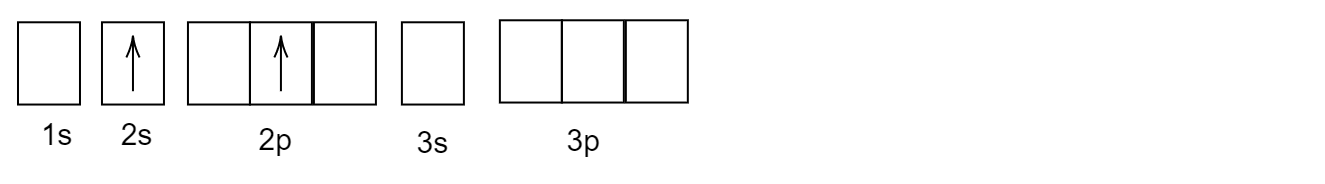
Complete answer:
An energy is required to excite an electron from its ground state to higher energy state i.e. excited state. The last electron means the valence electron is in a higher energy orbital, the element is state to be excited. The valence electrons are present in its ground state for a neutral element.
In the given question, first look at the ground state configuration for helium \[He\] . The atomic number of helium is two and its electronic configuration is \[1{s^2}\]. Therefore, two electrons are present in \[1s\] orbital.
Now, after the first excitation of valence electrons, one electron from \[1s\] orbital will jump to \[2p\] orbital as given in the question.
After its first excitation, when the atom of helium \[He\] is excited, the second electron absorbs the energy and gets excited and goes to a higher energy level. But, the first electron which was excited earlier remains back in the \[1s\] orbital.
The correct answer is option (B).
Note:
It is noted that an excited state atom is an atom in which the total energy of the electron can be lowered by transferring one or more electrons into different orbitals. Excited state usually exists for a short period of time. Long lived excited states are called metastable.

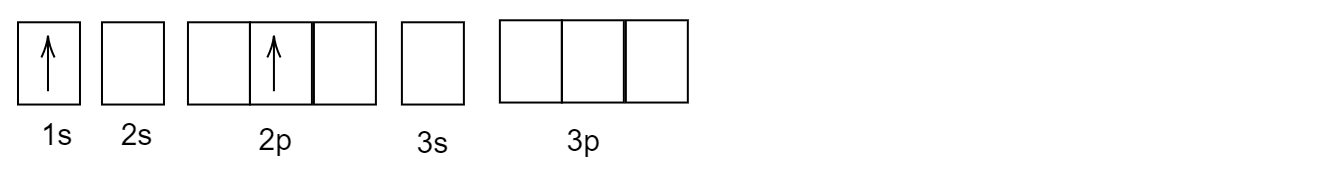
Complete answer:
An energy is required to excite an electron from its ground state to higher energy state i.e. excited state. The last electron means the valence electron is in a higher energy orbital, the element is state to be excited. The valence electrons are present in its ground state for a neutral element.
In the given question, first look at the ground state configuration for helium \[He\] . The atomic number of helium is two and its electronic configuration is \[1{s^2}\]. Therefore, two electrons are present in \[1s\] orbital.
Now, after the first excitation of valence electrons, one electron from \[1s\] orbital will jump to \[2p\] orbital as given in the question.

After its first excitation, when the atom of helium \[He\] is excited, the second electron absorbs the energy and gets excited and goes to a higher energy level. But, the first electron which was excited earlier remains back in the \[1s\] orbital.
The correct answer is option (B).
Note:
It is noted that an excited state atom is an atom in which the total energy of the electron can be lowered by transferring one or more electrons into different orbitals. Excited state usually exists for a short period of time. Long lived excited states are called metastable.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




