
The electrolyte in dry cell is -
(A) Copper sulphate
(B) Zinc sulphate
(C) Sulphuric acid
(D) Ammonium chloride
Answer
586.2k+ views
Hint
Generally, the dry cell consists of the electrolyte which is in the form of paste. The moisture which is present in the paste electrolyte is enough to allow the current to flow between two electrodes. The dry cell comprises zinc anode and carbon cathode.
Complete step by step solution
A battery or cell is a device which consists of one or more electrochemical cells that are used for converting the chemical energy to electrical energy. The dry is commonly used for general and daily purposes. A dry cell commonly uses the electrolyte of immobilisation as a paste, which has some moisture in it, and that moisture is enough to pass the charges from one electrode to another electrode. In dry cells the electrolyte does not spill out when we use the dry cell in any orientation. But the wet cell spills the electrolyte out when it has some angle of inclination.
This is the main reason that the paste-like electrolyte is used in the dry cell. Most commonly the dry cell battery is the zinc carbon battery. And the cell is made up of an outer container, one anode, one cathode and electrolyte are filled in the container.
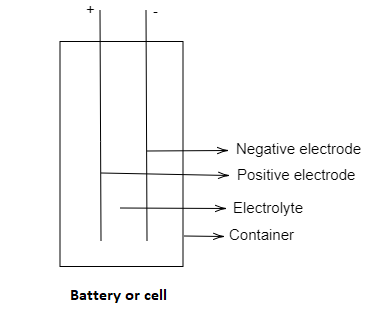
Thus, the electrolyte used in the dry cell is ammonium chloride.
Hence, the option (D) is the correct answer.
Note
By knowing the physical states of the elements which are given in the options, then the solution can be determined. And also know the difference between dry cell and wet cell. And understanding the application of the dry cell also helps to find the solution.
Generally, the dry cell consists of the electrolyte which is in the form of paste. The moisture which is present in the paste electrolyte is enough to allow the current to flow between two electrodes. The dry cell comprises zinc anode and carbon cathode.
Complete step by step solution
A battery or cell is a device which consists of one or more electrochemical cells that are used for converting the chemical energy to electrical energy. The dry is commonly used for general and daily purposes. A dry cell commonly uses the electrolyte of immobilisation as a paste, which has some moisture in it, and that moisture is enough to pass the charges from one electrode to another electrode. In dry cells the electrolyte does not spill out when we use the dry cell in any orientation. But the wet cell spills the electrolyte out when it has some angle of inclination.
This is the main reason that the paste-like electrolyte is used in the dry cell. Most commonly the dry cell battery is the zinc carbon battery. And the cell is made up of an outer container, one anode, one cathode and electrolyte are filled in the container.

Thus, the electrolyte used in the dry cell is ammonium chloride.
Hence, the option (D) is the correct answer.
Note
By knowing the physical states of the elements which are given in the options, then the solution can be determined. And also know the difference between dry cell and wet cell. And understanding the application of the dry cell also helps to find the solution.
Recently Updated Pages
Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Computer Science: Engaging Questions & Answers for Success

Class 10 Question and Answer - Your Ultimate Solutions Guide

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Trending doubts
A boat goes 24 km upstream and 28 km downstream in class 10 maths CBSE

State and explain Ohms law class 10 physics CBSE

Write a letter to the editor of a newspaper explaining class 10 english CBSE

Distinguish between soap and detergent class 10 chemistry CBSE

a Why did Mendel choose pea plants for his experiments class 10 biology CBSE

What is a "free hit" awarded for in limited-overs cricket?




