
The edge length of sodium chloride unit cell is \[564{\text{ }}pm\]. If the size of \[{\text{C}}{{\text{l}}^{\text{ - }}}\] ion is \[181{\text{ }}pm\]. The size of \[{\text{N}}{{\text{a}}^{\text{ + }}}\] ion will be:-
A. 101
B. 181
C. 410
D.202
Answer
591.3k+ views
Hint: We must know that the structure of \[{\text{NaCl}}\] crystal is face-centred cubic (FCC) type cubic lattice. In the (FCC) arrangement, there is one additional atom at the centre of each of the six faces of the unit cube.
Complete step by step answer:
See the figure; we can clearly see that, each edge contains two \[{\text{C}}{{\text{l}}^{\text{ - }}}\]ions and one \[{\text{N}}{{\text{a}}^{\text{ + }}}\] ion.
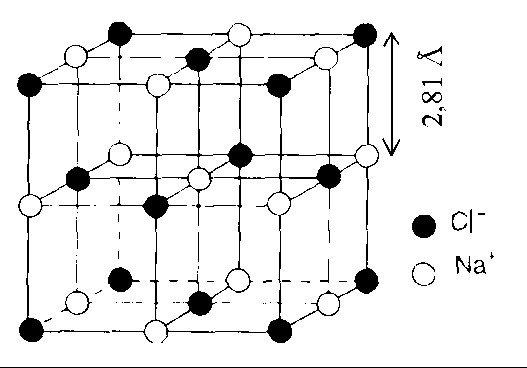
So, we know that the edge length = \[\]${\text{rN}}{{\text{a}}^{\text{ + }}}{\text{ + 2rC}}{{\text{l}}^{\text{ - }}}{\text{ + rN}}{{\text{a}}^{\text{ + }}}$
Or, edge length = ${\text{2rN}}{{\text{a}}^{\text{ + }}}{\text{ + 2rC}}{{\text{l}}^{\text{ - }}}$
In the question, they given edge length = \[{\text{564pm}}\]
Also ionic radius of sodium, ${\text{rC}}{{\text{l}}^{\text{ - }}}$\[{\text{ = 181pm}}\]
\[2rC{l^ - } = 2 \times 181 = 362\]
Now, we can substitute the values in edge length equation,
\[{\text{564pm = 2rN}}{{\text{a}}^{\text{ + }}}{\text{ + 362}}\]
\[
{\text{564pm - 362pm = 2rC}}{{\text{l}}^{\text{ - }}} \\
{\text{202pm = 2rC}}{{\text{l}}^{\text{ - }}} \\
{\text{rC}}{{\text{l}}^ - } = \dfrac{{{\text{202pm }}}}{2} = 101{\text{pm}} \\
\]
So, we found the ionic radius of sodium ion is\[{\text{101 pm}}\].
Hence, the correct option is option A.
Additional information:
1. Lattice structures are three-dimensional structures composed of one or more repeating unit cells. We can classify the cubic lattices as three types such as simple cubic (SC), body-centred cubic (BCC), and face-centred cubic (FCC).
2. The FCC has a coordination number of 12 and contains 4 atoms per unit cell. The BCC has a coordination number of 8 and contains 2 atoms per unit cell. The simple cubic has a coordination number of 6 and contains 1 atom per unit cell.
3. Sodium chloride has a molar mass of \[58.44{\text{ }}g/mol\]. It is an ionic compound consisting of a sodium cation (\[N{a^ + }\]) and a chloride anion (\[C{l^ - }\]).
The following are the more details of structure of sodium chloride
Lattice Type: Face-centred
Crystal System: Cubic
Cell Parameters: a = \[5.6402{\text{ }}{A},{\text{ }}Z = 4\]
Alternate Names: Halite, rock salt, sea salt, table
Note:
The equation for edge length calculation comes from the Pythogrean theorem .
We know this: \[{{\text{d}}^{\text{2}}}{\text{ + }}{{\text{d}}^{\text{2}}}{\text{ = }}{\left( {{\text{4r}}} \right)^{\text{2}}}\],
Where, ‘d’ is the edge length and ‘r’ is the radius of the atom.
Complete step by step answer:
See the figure; we can clearly see that, each edge contains two \[{\text{C}}{{\text{l}}^{\text{ - }}}\]ions and one \[{\text{N}}{{\text{a}}^{\text{ + }}}\] ion.

So, we know that the edge length = \[\]${\text{rN}}{{\text{a}}^{\text{ + }}}{\text{ + 2rC}}{{\text{l}}^{\text{ - }}}{\text{ + rN}}{{\text{a}}^{\text{ + }}}$
Or, edge length = ${\text{2rN}}{{\text{a}}^{\text{ + }}}{\text{ + 2rC}}{{\text{l}}^{\text{ - }}}$
In the question, they given edge length = \[{\text{564pm}}\]
Also ionic radius of sodium, ${\text{rC}}{{\text{l}}^{\text{ - }}}$\[{\text{ = 181pm}}\]
\[2rC{l^ - } = 2 \times 181 = 362\]
Now, we can substitute the values in edge length equation,
\[{\text{564pm = 2rN}}{{\text{a}}^{\text{ + }}}{\text{ + 362}}\]
\[
{\text{564pm - 362pm = 2rC}}{{\text{l}}^{\text{ - }}} \\
{\text{202pm = 2rC}}{{\text{l}}^{\text{ - }}} \\
{\text{rC}}{{\text{l}}^ - } = \dfrac{{{\text{202pm }}}}{2} = 101{\text{pm}} \\
\]
So, we found the ionic radius of sodium ion is\[{\text{101 pm}}\].
Hence, the correct option is option A.
Additional information:
1. Lattice structures are three-dimensional structures composed of one or more repeating unit cells. We can classify the cubic lattices as three types such as simple cubic (SC), body-centred cubic (BCC), and face-centred cubic (FCC).
2. The FCC has a coordination number of 12 and contains 4 atoms per unit cell. The BCC has a coordination number of 8 and contains 2 atoms per unit cell. The simple cubic has a coordination number of 6 and contains 1 atom per unit cell.
3. Sodium chloride has a molar mass of \[58.44{\text{ }}g/mol\]. It is an ionic compound consisting of a sodium cation (\[N{a^ + }\]) and a chloride anion (\[C{l^ - }\]).
The following are the more details of structure of sodium chloride
Lattice Type: Face-centred
Crystal System: Cubic
Cell Parameters: a = \[5.6402{\text{ }}{A},{\text{ }}Z = 4\]
Alternate Names: Halite, rock salt, sea salt, table
Note:
The equation for edge length calculation comes from the Pythogrean theorem .
We know this: \[{{\text{d}}^{\text{2}}}{\text{ + }}{{\text{d}}^{\text{2}}}{\text{ = }}{\left( {{\text{4r}}} \right)^{\text{2}}}\],
Where, ‘d’ is the edge length and ‘r’ is the radius of the atom.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




