
The distinguishing test for triple bond containing acidic hydrogen is
A.${\rm{Ag}}\left( {{\rm{N}}{{\rm{H}}_{\rm{3}}}} \right)_2^ + $
B. ${\rm{B}}{{\rm{r}}_{\rm{2}}}$ in ${\rm{CC}}{{\rm{l}}_{\rm{4}}}$
C. Alkaline ${\rm{KMn}}{{\rm{O}}_{\rm{4}}}$
D.${\rm{AlC}}{{\rm{l}}_{\rm{3}}}$
Answer
578.1k+ views
Hint: We know that Tollen's reagent is an ammoniacal solution of silver nitrate. ${\rm{Ag}}\left( {{\rm{N}}{{\rm{H}}_{\rm{3}}}} \right)_2^ + $ is prepared by reacting silver nitrate $\left( {{\rm{AgN}}{{\rm{O}}_{\rm{3}}}} \right)$and liquid ammonia $\left( {{\rm{N}}{{\rm{H}}_{\rm{3}}}} \right)$. This reagent is used in confirming aldehyde groups in a compound.
Complete step by step answer:
Let’s identify the correct answer from the options.
Option A says ${\rm{Ag}}\left( {{\rm{N}}{{\rm{H}}_{\rm{3}}}} \right)_2^ + $ distinguishes triple bond possessing acidic hydrogen. We know that Tollen's reagent reacts on terminal alkynes and replaces the terminal hydrogen atom with silver (Ag) and produces white colored precipitate. But, Tollen’s reagent does not react with alkenes. The reaction can be shown as below:
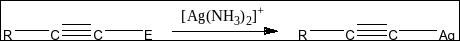
Option B says ${\rm{B}}{{\rm{r}}_{\rm{2}}}$ in ${\rm{CC}}{{\rm{l}}_{\rm{4}}}$ distinguishes triple bond possessing acidic hydrogen. This is not correct as alkene and alkyne both reacts with ${\rm{B}}{{\rm{r}}_{\rm{2}}}$ in presence of ${\rm{CC}}{{\rm{l}}_{\rm{4}}}$ to give dihalogen product. So, B is not the correct answer.
Option C says alkaline ${\rm{KMn}}{{\rm{O}}_{\rm{4}}}$ distinguishes triple bonds possessing acidic hydrogen. We know that alkaline potassium permanganate is an oxidizing agent. It reacts with terminal alkyne to form carboxylic acid. It also reacts with alkene to form vicinal diol. So, terminal hydrogen is not distinguishable by ${\rm{KMn}}{{\rm{O}}_{\rm{4}}}$.
Option D says ${\rm{AlC}}{{\rm{l}}_{\rm{3}}}$ distinguishes triple bond possessing acidic hydrogen. We know that, ${\rm{AlC}}{{\rm{l}}_{\rm{3}}}$ helps in chlorination in aromatic molecules in addition to chlorine. So, it does not distinguish triple bonds possessing acidic hydrogen.
Therefore, we can say that only Tollen’s reagent $\left( {{\rm{Ag}}\left( {{\rm{N}}{{\rm{H}}_{\rm{3}}}} \right)_2^ + } \right)$ among the options can distinguishes triple bond possessing acidic hydrogen. Hence, the correct answer is option A.
Note:
Tollen’s reagent is used for confirmation of aldehyde functional groups in a compound. All aldehydes give positive Tollens tests. All ketones except $\alpha $ -hydroxy ketones do not react with Tollen’s reagent.
Complete step by step answer:
Let’s identify the correct answer from the options.
Option A says ${\rm{Ag}}\left( {{\rm{N}}{{\rm{H}}_{\rm{3}}}} \right)_2^ + $ distinguishes triple bond possessing acidic hydrogen. We know that Tollen's reagent reacts on terminal alkynes and replaces the terminal hydrogen atom with silver (Ag) and produces white colored precipitate. But, Tollen’s reagent does not react with alkenes. The reaction can be shown as below:
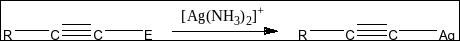
Option B says ${\rm{B}}{{\rm{r}}_{\rm{2}}}$ in ${\rm{CC}}{{\rm{l}}_{\rm{4}}}$ distinguishes triple bond possessing acidic hydrogen. This is not correct as alkene and alkyne both reacts with ${\rm{B}}{{\rm{r}}_{\rm{2}}}$ in presence of ${\rm{CC}}{{\rm{l}}_{\rm{4}}}$ to give dihalogen product. So, B is not the correct answer.
Option C says alkaline ${\rm{KMn}}{{\rm{O}}_{\rm{4}}}$ distinguishes triple bonds possessing acidic hydrogen. We know that alkaline potassium permanganate is an oxidizing agent. It reacts with terminal alkyne to form carboxylic acid. It also reacts with alkene to form vicinal diol. So, terminal hydrogen is not distinguishable by ${\rm{KMn}}{{\rm{O}}_{\rm{4}}}$.
Option D says ${\rm{AlC}}{{\rm{l}}_{\rm{3}}}$ distinguishes triple bond possessing acidic hydrogen. We know that, ${\rm{AlC}}{{\rm{l}}_{\rm{3}}}$ helps in chlorination in aromatic molecules in addition to chlorine. So, it does not distinguish triple bonds possessing acidic hydrogen.
Therefore, we can say that only Tollen’s reagent $\left( {{\rm{Ag}}\left( {{\rm{N}}{{\rm{H}}_{\rm{3}}}} \right)_2^ + } \right)$ among the options can distinguishes triple bond possessing acidic hydrogen. Hence, the correct answer is option A.
Note:
Tollen’s reagent is used for confirmation of aldehyde functional groups in a compound. All aldehydes give positive Tollens tests. All ketones except $\alpha $ -hydroxy ketones do not react with Tollen’s reagent.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




