
The degree of dissociation of $ S{O_3} $ is $ \alpha $ at equilibrium pressure $ {P_0} $ . What is $ {K_p} $ for the reaction $ 2S{O_3}\left( g \right) \rightleftarrows 2S{O_2}\left( g \right) + {O_2}\left( g \right) $ ?
A) $ \dfrac{{{P_0}{\alpha ^3}}}{{2{{\left( {1 - \alpha } \right)}^3}}} $
B) $ \dfrac{{{P_0}{\alpha ^3}}}{{\left( {2 + \alpha } \right){{\left( {1 - \alpha } \right)}^2}}} $
C) $ \dfrac{{{P_0}{\alpha ^3}}}{{2{{\left( {1 - \alpha } \right)}^2}}} $
D) None of the above.
Answer
543.9k+ views
Hint :In the given reaction, Sulfur trioxide is dissociating to form Sulfur dioxide and Oxygen. The total pressure of this reaction is given as $ {P_0} $ so find the partial pressures of each compound. The formula of $ {K_p} $ is the ratio of partial pressures of products to the reactants.
Complete Step By Step Answer:
Given to us is a reaction involving the dissociation of Sulfur trioxide to Sulfur dioxide and Oxygen. The equation for this reaction is written as $ 2S{O_3}\left( g \right) \rightleftarrows 2S{O_2}\left( g \right) + {O_2}\left( g \right) $
This is an equilibrium reaction. Let the initial partial pressure of Sulfur trioxide be P. We can now write a table showing the partial pressures of products and reactants initially and at equilibrium.
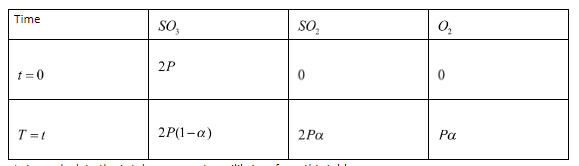
Let us calculate the total pressure at equilibrium from this table.
$ {P_{total}} = 2P\left( {1 - \alpha } \right) + 2P\alpha + P\alpha $
By solving, we get $ {P_{total}} = 2P - 2P\alpha + 2P\alpha + P\alpha = 2P + P\alpha $
It is already given to us that the total pressure at equilibrium is $ {P_0} $
By equating these two, we get $ {P_0} = P\left( {2 + \alpha } \right) $ and we can write this as $ P = \dfrac{{{P_0}}}{{2 + \alpha }} $
Now we write the formula of $ {K_p} $ for the given equilibrium reaction as follows:
$ {K_p} = \dfrac{{{{\left[ {{P_{S{O_2}}}} \right]}^2}\left[ {{P_{{O_2}}}} \right]}}{{{{\left[ {{P_{S{O_3}}}} \right]}^2}}} $
By substituting the values of partial pressures of each component, we get $ {K_p} = \dfrac{{{{\left( {2P\alpha } \right)}^2}\left( {P\alpha } \right)}}{{{{\left( {2P\left( {1 - \alpha } \right)} \right)}^2}}} $
On solving, we get $ {K_p} = \dfrac{{4{P^3}{\alpha ^3}}}{{4{P^2}{{\left( {1 - \alpha } \right)}^2}}} = \dfrac{{P{\alpha ^3}}}{{{{\left( {1 - \alpha } \right)}^2}}} $
We have already found a relation between partial pressure P and total pressure $ {P_0} $ so let us substitute the value of P from that relation.
Now the above equation becomes $ {K_p} = \dfrac{{{P_0}{\alpha ^3}}}{{\left( {2 + \alpha } \right){{\left( {1 - \alpha } \right)}^2}}} $
Therefore the correct answer is option B.
Note :
It is to be noted that before writing the formula for $ {K_p} $ or drawing the table for partial pressures of the compounds, the reaction equation must be balanced. This is because $ {K_p} $ is dependent on the exponential values of the partial pressures.
Complete Step By Step Answer:
Given to us is a reaction involving the dissociation of Sulfur trioxide to Sulfur dioxide and Oxygen. The equation for this reaction is written as $ 2S{O_3}\left( g \right) \rightleftarrows 2S{O_2}\left( g \right) + {O_2}\left( g \right) $
This is an equilibrium reaction. Let the initial partial pressure of Sulfur trioxide be P. We can now write a table showing the partial pressures of products and reactants initially and at equilibrium.

Let us calculate the total pressure at equilibrium from this table.
$ {P_{total}} = 2P\left( {1 - \alpha } \right) + 2P\alpha + P\alpha $
By solving, we get $ {P_{total}} = 2P - 2P\alpha + 2P\alpha + P\alpha = 2P + P\alpha $
It is already given to us that the total pressure at equilibrium is $ {P_0} $
By equating these two, we get $ {P_0} = P\left( {2 + \alpha } \right) $ and we can write this as $ P = \dfrac{{{P_0}}}{{2 + \alpha }} $
Now we write the formula of $ {K_p} $ for the given equilibrium reaction as follows:
$ {K_p} = \dfrac{{{{\left[ {{P_{S{O_2}}}} \right]}^2}\left[ {{P_{{O_2}}}} \right]}}{{{{\left[ {{P_{S{O_3}}}} \right]}^2}}} $
By substituting the values of partial pressures of each component, we get $ {K_p} = \dfrac{{{{\left( {2P\alpha } \right)}^2}\left( {P\alpha } \right)}}{{{{\left( {2P\left( {1 - \alpha } \right)} \right)}^2}}} $
On solving, we get $ {K_p} = \dfrac{{4{P^3}{\alpha ^3}}}{{4{P^2}{{\left( {1 - \alpha } \right)}^2}}} = \dfrac{{P{\alpha ^3}}}{{{{\left( {1 - \alpha } \right)}^2}}} $
We have already found a relation between partial pressure P and total pressure $ {P_0} $ so let us substitute the value of P from that relation.
Now the above equation becomes $ {K_p} = \dfrac{{{P_0}{\alpha ^3}}}{{\left( {2 + \alpha } \right){{\left( {1 - \alpha } \right)}^2}}} $
Therefore the correct answer is option B.
Note :
It is to be noted that before writing the formula for $ {K_p} $ or drawing the table for partial pressures of the compounds, the reaction equation must be balanced. This is because $ {K_p} $ is dependent on the exponential values of the partial pressures.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




