
The decreasing order of electrical conductivity of the following aqueous solutions is:
0.1 M Formic acid (A)
0.1 M Acetic acid (B)
0.1 M Benzoic acid (C)
A. $\text{C}>\text{A}>\text{B}$
B. $\text{A}>\text{B}>\text{C}$
C. $\text{A}>\text{C}>\text{B}$
D. $\text{C}>\text{A}>\text{B}$
Answer
591.9k+ views
Hint: The electrical conductivity depends on the number of ions present in the solution that carries the current within it. The number of ions are decided by the ionizable particles or molarity of any solution. If the molarities of all the solutions given are the same, then the power of dissociation of the substance comes into action. More the strength of substance, more will be the electrical conductivity.
Complete answer:
In the question, the substances given are acids and the molarities of all three solutions are the same. So, let us now find the strength of all the three acids.
The strength of any acid is decided by its ability to produce hydrogen ions or $\left[ {{\text{H}}^{+}} \right]$ ions. The production of $\left[ {{\text{H}}^{+}} \right]$ ions will be further checked by resonance and inductive effect in their respective structures. So, let us study the structure of each acid one by one along with their strengths.
(A) Formic acid- This acid has one carbon atom and is the simplest carboxylic acid. Formic acid is also known as formaldehyde. It structure is
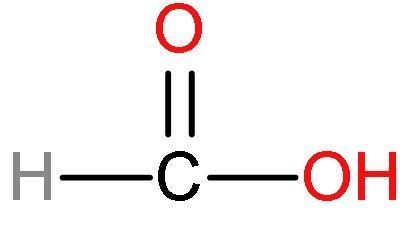
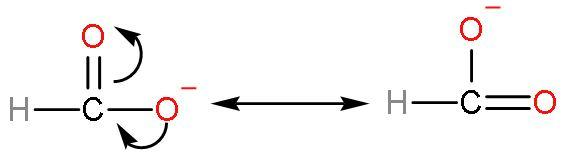
Formic acid is much acidic because there is such no presence of methyl groups in this acid. No methyl groups so, no inductive effect. When species have the tendency to donate electrons such as a methyl group to a carbon chain, this charge is relayed through the chain and the effect is called the positive Inductive Effect. It has good acidic strength. As, after losing $\left[ {{\text{H}}^{+}} \right]$ ions, it is highly stable due to highly stabilised resonating structures.
(B) Acetic acid- It is the second member of the carboxylic group as it has two carbon atoms. It is also known as acetaldehyde. Its structure is
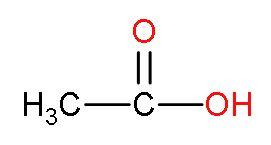
Methyl group has +I effect or denotes its electrons to the attached elements. This positive inductive effect neutralises the polarity of $-\text{COOH}$ bond. Thus, breaking of $-\text{COOH}$ bonds becomes difficult and thus producing less $\left[ {{\text{H}}^{+}} \right]$ ions. So, have low acidic strength.

(C) Benzoic acid- Benzoic acid has a carboxylic acid group attached to the benzene ring. It undergoes resonance with benzene and $-\text{COOH}$group.

The phenyl group shows –I effect which attracts the electrons thus, helping in increasing the polarity of $-\text{COOH}$ bond for easy ionization of $\left[ {{\text{H}}^{+}} \right]$ ions.
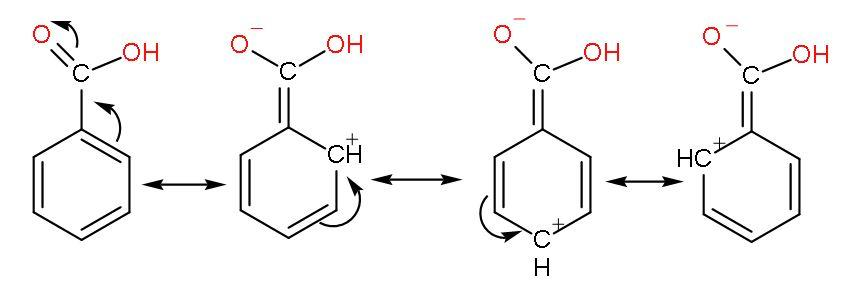
But its resonance with benzene rings forms unstable carbocations due to incomplete octet of carbon atoms (have only 6 electrons) makes benzoic acid less acidic than formic acid.
So, the order of acidic strength is $\text{Formic acid }>\text{Benzoic acid }>\text{Acetic acid}$. Hence, the order of electrical conductivity will be $\text{A > C > B}$ So, the correct answer is “Option C”.
Note: It is generally thought that aliphatic carboxylic acids are stronger acids than aromatic carboxylic acids due to resonating structures within the benzene ring. But here, benzoic acid is more acidic than acetic acid. It is because the inductive effect by the alkyl group on acetic acid destabilises the acetate ion whereas the benzene rings pull electrons away from groups attached to it.
Complete answer:
In the question, the substances given are acids and the molarities of all three solutions are the same. So, let us now find the strength of all the three acids.
The strength of any acid is decided by its ability to produce hydrogen ions or $\left[ {{\text{H}}^{+}} \right]$ ions. The production of $\left[ {{\text{H}}^{+}} \right]$ ions will be further checked by resonance and inductive effect in their respective structures. So, let us study the structure of each acid one by one along with their strengths.
(A) Formic acid- This acid has one carbon atom and is the simplest carboxylic acid. Formic acid is also known as formaldehyde. It structure is

Formic acid is much acidic because there is such no presence of methyl groups in this acid. No methyl groups so, no inductive effect. When species have the tendency to donate electrons such as a methyl group to a carbon chain, this charge is relayed through the chain and the effect is called the positive Inductive Effect. It has good acidic strength. As, after losing $\left[ {{\text{H}}^{+}} \right]$ ions, it is highly stable due to highly stabilised resonating structures.

(B) Acetic acid- It is the second member of the carboxylic group as it has two carbon atoms. It is also known as acetaldehyde. Its structure is

Methyl group has +I effect or denotes its electrons to the attached elements. This positive inductive effect neutralises the polarity of $-\text{COOH}$ bond. Thus, breaking of $-\text{COOH}$ bonds becomes difficult and thus producing less $\left[ {{\text{H}}^{+}} \right]$ ions. So, have low acidic strength.

(C) Benzoic acid- Benzoic acid has a carboxylic acid group attached to the benzene ring. It undergoes resonance with benzene and $-\text{COOH}$group.

The phenyl group shows –I effect which attracts the electrons thus, helping in increasing the polarity of $-\text{COOH}$ bond for easy ionization of $\left[ {{\text{H}}^{+}} \right]$ ions.

But its resonance with benzene rings forms unstable carbocations due to incomplete octet of carbon atoms (have only 6 electrons) makes benzoic acid less acidic than formic acid.
So, the order of acidic strength is $\text{Formic acid }>\text{Benzoic acid }>\text{Acetic acid}$. Hence, the order of electrical conductivity will be $\text{A > C > B}$ So, the correct answer is “Option C”.
Note: It is generally thought that aliphatic carboxylic acids are stronger acids than aromatic carboxylic acids due to resonating structures within the benzene ring. But here, benzoic acid is more acidic than acetic acid. It is because the inductive effect by the alkyl group on acetic acid destabilises the acetate ion whereas the benzene rings pull electrons away from groups attached to it.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




