
The curve showing the variation of pressure with temperature for a given amount of adsorption is called-
A.Adsorption isobar
B.Adsorption isotherm
C.Adsorption isostere
D.Adsorption isochore

Answer
582.6k+ views
HintAdsorption is the phenomenon in which the higher concentration of molecular species whether it is gas or liquid remains on the surface of the solid and does not penetrate the bulk of the solid. They are of two types-(i) Physisorption in which the particles are held on the surface by physical force like vanderwaals (ii) Chemisorption in which the molecules are held at the surface by forces similar to chemical bonds.
Step-by-Step Solution
Pressure and temperature are two main factors that influence the adsorption of a gas on a solid surface.
1. Adsorption isobar- shows the effect of temperature on adsorption of a gas on the surface of solid. Isobar means the same pressure which means pressure is kept constant. Adsorption isobars are plotted at constant pressure. It is plotted for both physical and chemical adsorption.
2.Adsorption isotherm- Isotherm means same temperature. Adsorption isotherms are plotted between the amount of gas adsorbed and the corresponding pressure at constant temperature.
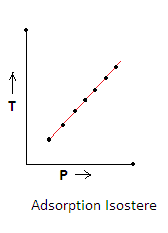
3.Adsorption Isostere- It is a plot of temperature versus pressure for a given amount of adsorption. In this graph amount of gas adsorbed is kept constant.It shows that linear relationship exists between temperature and pressure and is given as-
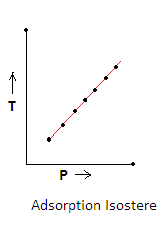
a.When the temperature increases the extent of adsorption decreases.
b.So to get the same amount of adsorption at a higher temperature, pressure is also increased.
4.Adsorption Isochore – It is the plot between temperature and density at constant volume. Isochore means volume is kept constant or the same.
Note: Adsorption process is used in various chemical processes in laboratories and in industry. The uses of adsorption are-
1.In gas masks activated charcoal is used to absorb poisonous and toxic gases.
2.Mordants are used in dying clothes as they absorb the dye.
3.In chromatography adsorption is used to purify and separate substances from their compounds.
4.Charcoal is used to remove traces of air to create vacuum in the vacuum pump.
5.In removal of coloring from sugar, juice and vegetable oils by using fuller’s earth or activated charcoal.
Step-by-Step Solution
Pressure and temperature are two main factors that influence the adsorption of a gas on a solid surface.
1. Adsorption isobar- shows the effect of temperature on adsorption of a gas on the surface of solid. Isobar means the same pressure which means pressure is kept constant. Adsorption isobars are plotted at constant pressure. It is plotted for both physical and chemical adsorption.
2.Adsorption isotherm- Isotherm means same temperature. Adsorption isotherms are plotted between the amount of gas adsorbed and the corresponding pressure at constant temperature.
3.Adsorption Isostere- It is a plot of temperature versus pressure for a given amount of adsorption. In this graph amount of gas adsorbed is kept constant.It shows that linear relationship exists between temperature and pressure and is given as-

a.When the temperature increases the extent of adsorption decreases.
b.So to get the same amount of adsorption at a higher temperature, pressure is also increased.
4.Adsorption Isochore – It is the plot between temperature and density at constant volume. Isochore means volume is kept constant or the same.
Note: Adsorption process is used in various chemical processes in laboratories and in industry. The uses of adsorption are-
1.In gas masks activated charcoal is used to absorb poisonous and toxic gases.
2.Mordants are used in dying clothes as they absorb the dye.
3.In chromatography adsorption is used to purify and separate substances from their compounds.
4.Charcoal is used to remove traces of air to create vacuum in the vacuum pump.
5.In removal of coloring from sugar, juice and vegetable oils by using fuller’s earth or activated charcoal.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




