
The covalency of nitrogen in $ HN{{O}_{3}} $ is:
(A) $ 0 $
(B) $ 3 $
(C) $ 4 $
(D) $ 5 $
Answer
529.2k+ views
Hint : We know that covalence of an element is the number of bonds that an element makes within a compound or molecule. In $ HN{{O}_{3}} $ , nitrogen is attached to two oxygen atoms and one hydroxide ion as we can see from the structure of $ HN{{O}_{3}} $ and hence we have to count total bonds around nitrogen in $ HN{{O}_{3}} $ in order to know its covalence in $ HN{{O}_{3}} $ .
Complete Step By Step Answer:
The electronic configuration of nitrogen is $ 1{{s}^{2}}2{{s}^{2}}2{{p}^{3}} $ . The orbital $ 1s $ is completely filled and is present quite inside the shell and hence cannot be used in bonding. Only 2s and 2p orbitals are left. The formation of four bonds by nitrogen (or nitrogen having a covalency of four) can be explained by having a hybridization of $ s{{p}^{3}} $ by combination of 2s and 2p orbitals. Nitrogen uses its lone pair of electrons to have a covalence of four. The dot structure is given as;
$ \underset{\scriptscriptstyle\centerdot\centerdot}{\ddot{O}}::N(:\underset{\scriptscriptstyle\centerdot\centerdot}{\ddot{O}}:):\underset{\scriptscriptstyle\centerdot\centerdot}{\ddot{O}}:H $
Nitrogen having a covalency of three has a non-bonding or lone pair of electrons with it.
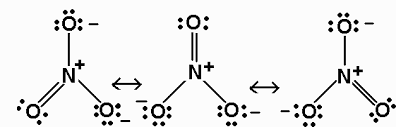 As we can see in the structure of $ HN{{O}_{3}} $ , nitrogen do not contain lone pair of electrons as a positive charge is present on nitrogen and hence the covalency of nitrogen is $ 4 $
As we can see in the structure of $ HN{{O}_{3}} $ , nitrogen do not contain lone pair of electrons as a positive charge is present on nitrogen and hence the covalency of nitrogen is $ 4 $
Hence, option C is the right answer.
Additional Information:
Since nitrogen does not possess d orbitals in its valence shell $ \left( n=2 \right) $ , therefore it can show a maximum covalency of $ 4 $ and that too when it donates its lone pair of electrons. In other words, nitrogen cannot extend its covalence beyond $ 4 $ . That is why nitrogen does not form $ N{{F}_{5}}\,or\,NC{{l}_{5}} $ .
Note :
It should be noted that phosphorus and other elements of the group $ 13 $ have empty d orbitals and can utilize all their valence orbitals to exhibit covalence of $ 5\,or\,6 $ . We should know the difference between oxidation state and covalency. The oxidation state of nitrogen in $ HN{{O}_{3}} $ is $ +5 $ while the covalency of nitrogen in $ HN{{O}_{3}} $ is four.
Complete Step By Step Answer:
The electronic configuration of nitrogen is $ 1{{s}^{2}}2{{s}^{2}}2{{p}^{3}} $ . The orbital $ 1s $ is completely filled and is present quite inside the shell and hence cannot be used in bonding. Only 2s and 2p orbitals are left. The formation of four bonds by nitrogen (or nitrogen having a covalency of four) can be explained by having a hybridization of $ s{{p}^{3}} $ by combination of 2s and 2p orbitals. Nitrogen uses its lone pair of electrons to have a covalence of four. The dot structure is given as;
$ \underset{\scriptscriptstyle\centerdot\centerdot}{\ddot{O}}::N(:\underset{\scriptscriptstyle\centerdot\centerdot}{\ddot{O}}:):\underset{\scriptscriptstyle\centerdot\centerdot}{\ddot{O}}:H $
Nitrogen having a covalency of three has a non-bonding or lone pair of electrons with it.

Hence, option C is the right answer.
Additional Information:
Since nitrogen does not possess d orbitals in its valence shell $ \left( n=2 \right) $ , therefore it can show a maximum covalency of $ 4 $ and that too when it donates its lone pair of electrons. In other words, nitrogen cannot extend its covalence beyond $ 4 $ . That is why nitrogen does not form $ N{{F}_{5}}\,or\,NC{{l}_{5}} $ .
Note :
It should be noted that phosphorus and other elements of the group $ 13 $ have empty d orbitals and can utilize all their valence orbitals to exhibit covalence of $ 5\,or\,6 $ . We should know the difference between oxidation state and covalency. The oxidation state of nitrogen in $ HN{{O}_{3}} $ is $ +5 $ while the covalency of nitrogen in $ HN{{O}_{3}} $ is four.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




