
The correct sequences of reagents for the following conversion will be:
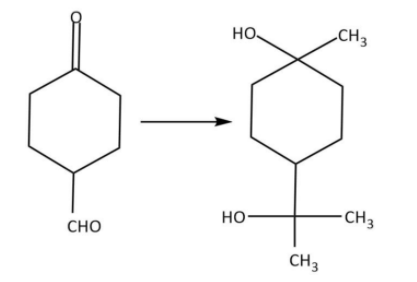
A. ${\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{MgBr}},{{\rm{H}}^ + }/{\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{OH}},{\left[ {{\rm{Ag}}{{\left( {{\rm{N}}{{\rm{H}}_{\rm{3}}}} \right)}_2}} \right]^ + }{\rm{O}}{{\rm{H}}^ - }$
B. ${\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{MgBr}},{\left[ {{\rm{Ag}}{{\left( {{\rm{N}}{{\rm{H}}_{\rm{3}}}} \right)}_2}} \right]^ + }{\rm{O}}{{\rm{H}}^ - },{{\rm{H}}^ + }/{\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{OH}}$
C. ${\left[ {{\rm{Ag}}{{\left( {{\rm{N}}{{\rm{H}}_{\rm{3}}}} \right)}_2}} \right]^ + }{\rm{O}}{{\rm{H}}^ - },{\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{MgBr}},{{\rm{H}}^ + }/{\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{OH}}$
D. ${\left[ {{\rm{Ag}}{{\left( {{\rm{N}}{{\rm{H}}_{\rm{3}}}} \right)}_2}} \right]^ + }{\rm{O}}{{\rm{H}}^ - },{{\rm{H}}^ + }/{\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{OH}},{\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{MgBr}}$

Answer
569.1k+ views
Hint: We can deduce the correct sequence by considering as to what each given reagent is used for and how it can be used here to bring out the specific conversion.
We are given three reagents as ${\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{MgBr}},{{\rm{H}}^ + }/{\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{OH}}$ and ${\left[ {{\rm{Ag}}{{\left( {{\rm{N}}{{\rm{H}}_{\rm{3}}}} \right)}_2}} \right]^ + }{\rm{O}}{{\rm{H}}^ - }$. Let’s talk about these briefly.
Complete answer
The first reagent is methyl magnesium bromide and can be recognized to be a Grignard reagent. We know that Grignard reagent has a nucleophilic carbon that is attached to magnesium and can be used to attack an electrophilic carbon. Our second reagent is acid and methanol taken together. We know that it can be used for esterification of a carboxylic acid. The last reagent is Tollen’s reagent that we can use for oxidizing an aldehyde to give a carboxylic acid.
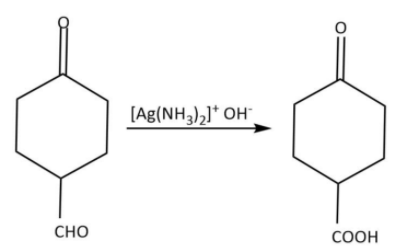
Now, let’s consider the reactant, it has a ketone as well as an aldehyde group. First of all, we will use the Tollen’s reagent for selective oxidation of aldehyde as follows:
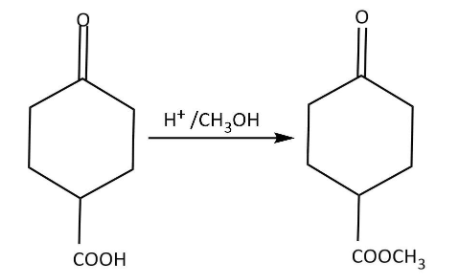
Now we can use the acid and methanol to carry out the esterification as follows:
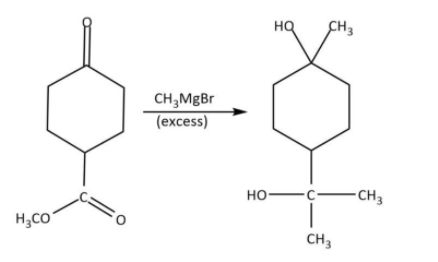
Finally, we will use the given Grignard reagent, methyl magnesium bromide to carry out the conversion as follows:
Hence, we have to use the given reagents in the following sequence: firstly, Tollen’s reagent, followed by acid with methanol and finally methyl magnesium bromide (Grignard reagent).
Hence, the correct option is D.
Note:
We can use Tollen’s reagent for differentiating between an aldehyde and ketone as it is a mild oxidizing agent and oxidized only aldehyde not ketone.
We are given three reagents as ${\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{MgBr}},{{\rm{H}}^ + }/{\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{OH}}$ and ${\left[ {{\rm{Ag}}{{\left( {{\rm{N}}{{\rm{H}}_{\rm{3}}}} \right)}_2}} \right]^ + }{\rm{O}}{{\rm{H}}^ - }$. Let’s talk about these briefly.
Complete answer
The first reagent is methyl magnesium bromide and can be recognized to be a Grignard reagent. We know that Grignard reagent has a nucleophilic carbon that is attached to magnesium and can be used to attack an electrophilic carbon. Our second reagent is acid and methanol taken together. We know that it can be used for esterification of a carboxylic acid. The last reagent is Tollen’s reagent that we can use for oxidizing an aldehyde to give a carboxylic acid.
Now, let’s consider the reactant, it has a ketone as well as an aldehyde group. First of all, we will use the Tollen’s reagent for selective oxidation of aldehyde as follows:

Now we can use the acid and methanol to carry out the esterification as follows:

Finally, we will use the given Grignard reagent, methyl magnesium bromide to carry out the conversion as follows:

Hence, we have to use the given reagents in the following sequence: firstly, Tollen’s reagent, followed by acid with methanol and finally methyl magnesium bromide (Grignard reagent).
Hence, the correct option is D.
Note:
We can use Tollen’s reagent for differentiating between an aldehyde and ketone as it is a mild oxidizing agent and oxidized only aldehyde not ketone.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
What are the major means of transport Explain each class 12 social science CBSE

India is a sovereign socialist secular democratic republic class 12 social science CBSE

How many states of matter are there in total class 12 chemistry CBSE

What are the advantages of vegetative propagation class 12 biology CBSE

Suicide bags of cells are aEndoplasmic reticulum bLysosome class 12 biology CBSE

What is the Full Form of PVC, PET, HDPE, LDPE, PP and PS ?




