
The correct sequence of bond length in a single bond, a double bond and triple bond of $C$ is:
A:

B:
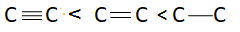
C:
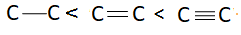
D:
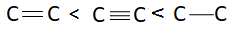

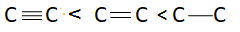
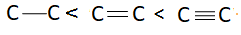
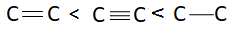
Answer
590.7k+ views
Hint: Bond length is the average distance between the nuclei of two bonded atoms. Bond length is different for different bonding atoms. Bond length is related to bond order, bond strength and bond dissociation energy. It depends upon the number of bonds between two atoms as well.
Complete step by step answer:
Bond length is the average distance between the nuclei of two bonded atoms. This length mainly depends on bond order. Bond order is calculated by dividing the number of bonds with total number of bonding pairs. In $C - C$ there is one bond and one bonding pair. This means bond order of $C - C$ is $1$. Similarly in
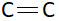 There are two bonds and one bonding pair. So, bond order of this molecule is $2$ (as bond order is$\dfrac{{{\text{number of bonds}}}}{{{\text{total bonding pairs}}}}$ ). In
There are two bonds and one bonding pair. So, bond order of this molecule is $2$ (as bond order is$\dfrac{{{\text{number of bonds}}}}{{{\text{total bonding pairs}}}}$ ). In
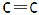 There are three bonds and one bonding pair therefore the bond order of this molecule is $3$. Bond length is inversely proportional to bond order. This means more is the bond order less is the bond length or less is the bond order more is the bond length. Bond order of triple bond $C$ is maximum therefore bond length of triple bond carbon is minimum and bond order of single bond carbon is minimum this means bond length of single bond carbon will be maximum. This means correct order of bond length will be:
There are three bonds and one bonding pair therefore the bond order of this molecule is $3$. Bond length is inversely proportional to bond order. This means more is the bond order less is the bond length or less is the bond order more is the bond length. Bond order of triple bond $C$ is maximum therefore bond length of triple bond carbon is minimum and bond order of single bond carbon is minimum this means bond length of single bond carbon will be maximum. This means correct order of bond length will be:
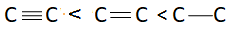
So, correct answer is option A.
Note:
Bond angle is the angle which is formed between two bonds. For the formation of bond angle there must be at least three atoms and two bonds between these atoms. Just like bond length, bond angle is also different for different molecules. It depends upon electronegativity, geometry and lone pairs on that molecule.
Complete step by step answer:
Bond length is the average distance between the nuclei of two bonded atoms. This length mainly depends on bond order. Bond order is calculated by dividing the number of bonds with total number of bonding pairs. In $C - C$ there is one bond and one bonding pair. This means bond order of $C - C$ is $1$. Similarly in
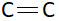
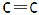
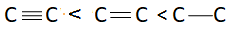
So, correct answer is option A.
Note:
Bond angle is the angle which is formed between two bonds. For the formation of bond angle there must be at least three atoms and two bonds between these atoms. Just like bond length, bond angle is also different for different molecules. It depends upon electronegativity, geometry and lone pairs on that molecule.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




