
What will be the correct order of the following:
A) Lone pair – Lone pair $ > $ Lone pair- bond pair $ > $ bond pair – bond pair
B) Lone pair – Lone pair $ > $ bond pair – bond pair $ > $ Lone pair- bond pair
C) Bond pair – bond pair $ > $ Lone pair- bond pair $ > $ Lone pair – Lone pair
D) Lone pair- bond pair $ > $ bond pair – bond pair $ > $ Lone pair – Lone pair
Answer
558.9k+ views
Hint: We will predict the bonding by understanding VSEPR theory. We know that the VSEPR theory is used to predict the shape of the molecules from the electron pairs that surround the central atoms of the molecule. the atoms tend to arrange themselves in such a manner that minimizes the repulsion between them.
Complete step-by-step answer:
So, the valence shell electron repulsion theory is the full form for VSEPR. It is fundamentally a model to calculate the number of atoms. Specifically, VSEPR models look at the bonding and molecular geometry of organic molecules and polyatomic ions and the strength of repulsion between a lone pair and a bond pair of electrons lies between the repulsion between two lone pairs and two bond pairs so the correct order will be:
Lone pair – Lone pair $ > $ Lone pair- bond pair $ > $ bond pair – bond pair
Each bond pair is shared between two ions and hence pulled in by two nuclei. Lone pair of electrons consumes more space than a bond pair of electrons.
Let us understand this concept by the help of an example:
In water molecules we know that two lone pairs are present on the central atom i.e., oxygen. So, there is a lone pair – bond pair repulsion between the oxygen and the hydrogen atoms which gives the molecule a bent shape or a V – shape with a bond angle of $104.5^\circ $
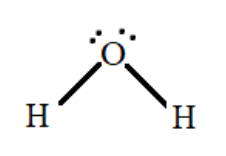
Hence the correct answer is Option A.
Note: Bond Angle is the angle between a bonded atom, the central atom, and another bonded atom. The shape of a molecule with only two toms is always linear. The Lone pair will occupy more space than a bond pair, hence when lone pair-lone pair will interact with each other they will repel more.
Complete step-by-step answer:
So, the valence shell electron repulsion theory is the full form for VSEPR. It is fundamentally a model to calculate the number of atoms. Specifically, VSEPR models look at the bonding and molecular geometry of organic molecules and polyatomic ions and the strength of repulsion between a lone pair and a bond pair of electrons lies between the repulsion between two lone pairs and two bond pairs so the correct order will be:
Lone pair – Lone pair $ > $ Lone pair- bond pair $ > $ bond pair – bond pair
Each bond pair is shared between two ions and hence pulled in by two nuclei. Lone pair of electrons consumes more space than a bond pair of electrons.
Let us understand this concept by the help of an example:
In water molecules we know that two lone pairs are present on the central atom i.e., oxygen. So, there is a lone pair – bond pair repulsion between the oxygen and the hydrogen atoms which gives the molecule a bent shape or a V – shape with a bond angle of $104.5^\circ $

Hence the correct answer is Option A.
Note: Bond Angle is the angle between a bonded atom, the central atom, and another bonded atom. The shape of a molecule with only two toms is always linear. The Lone pair will occupy more space than a bond pair, hence when lone pair-lone pair will interact with each other they will repel more.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




