
The correct order of priority to select functional group is:
(A)-${\text{RCHO > RCOR}}$
(B)-${\text{R - O - R > R - OH}}$
(C)-${\text{C}} \equiv {\text{C > C = C}}$
(D)-${\text{ - CHO > - COOH}}$
Answer
566.4k+ views
Hint: IUPAC has already proposed a series of priority of functional group and according to that functional groups with large no. of oxidizing agents will get high priority and as no. of oxidizing agent attach to the central atom of functional group decreases then priority of that functional group also decreases.
Complete step by step solution: Oxidizing agents are those agents which helps in the oxidation reaction by accepting electrons easily, and oxidizing property of any group is directly proportional to the electronegativity of the central atom present in the oxidizing agent.
-So, by keeping above concept in mind we can predict the highest and comparatively lowest priority of the functional group.
-In the option (A), aldehyde and ketone groups are given and aldehyde has higher priority than ketone group because in aldehyde with oxygen atom, hydrogen atom is present and in ketone with oxygen atom, carbon atom is present. So, oxygen of aldehyde is comparatively more electronegative due to hydrogen atom and showing appreciable oxidizing properties and gets highest priority.
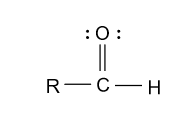
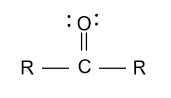
-In the option (B), ether and alcohol is given and alcohol has higher priority than ether group because in alcohol with oxygen atom, hydrogen atom is present and in ether with oxygen atom, 2 alkyl groups are present. So, oxygen of alcohol is comparatively more electronegative due to hydrogen atom and showing appreciable oxidizing properties and gets highest priority.

So, the order which is given in option (B) is wrong; and the correct order will be as ${\text{R - O - R < R - OH}}$.
-In the option (C), alkene and alkyne is given and alkyne has higher priority than alkene group in the given order which is wrong, because according to the series of functional groups given by IUPAC alkene has higher priority than alkyne.
So, the correct order for option (C) will be as ${\text{C}} \equiv {\text{C < C = C}}$.
-In the option (D), carboxylic acid and aldehyde is given and carboxylic acid has highest priority among all the functional groups in the chemistry because with carbon atom 2 strongly electronegative oxygen atoms are present.
So, the order which is given in option (D) is wrong; and the correct order will be as ${\text{ - CHO < - COOH}}$.
Hence, option (A) i.e. ${\text{RCHO > RCOR}}$ shows the correct order of priority to select a functional group.
Note: Here, in finding the highest priority of the functional group some of you may get confused between the term oxidizing agent and the oxidation because they sound similar, but they both deliver the opposite meaning. Oxidation stands for the removal of electrons and oxidizing agents stands for the accepting of electrons.
Complete step by step solution: Oxidizing agents are those agents which helps in the oxidation reaction by accepting electrons easily, and oxidizing property of any group is directly proportional to the electronegativity of the central atom present in the oxidizing agent.
-So, by keeping above concept in mind we can predict the highest and comparatively lowest priority of the functional group.
-In the option (A), aldehyde and ketone groups are given and aldehyde has higher priority than ketone group because in aldehyde with oxygen atom, hydrogen atom is present and in ketone with oxygen atom, carbon atom is present. So, oxygen of aldehyde is comparatively more electronegative due to hydrogen atom and showing appreciable oxidizing properties and gets highest priority.


-In the option (B), ether and alcohol is given and alcohol has higher priority than ether group because in alcohol with oxygen atom, hydrogen atom is present and in ether with oxygen atom, 2 alkyl groups are present. So, oxygen of alcohol is comparatively more electronegative due to hydrogen atom and showing appreciable oxidizing properties and gets highest priority.

So, the order which is given in option (B) is wrong; and the correct order will be as ${\text{R - O - R < R - OH}}$.
-In the option (C), alkene and alkyne is given and alkyne has higher priority than alkene group in the given order which is wrong, because according to the series of functional groups given by IUPAC alkene has higher priority than alkyne.
So, the correct order for option (C) will be as ${\text{C}} \equiv {\text{C < C = C}}$.
-In the option (D), carboxylic acid and aldehyde is given and carboxylic acid has highest priority among all the functional groups in the chemistry because with carbon atom 2 strongly electronegative oxygen atoms are present.
So, the order which is given in option (D) is wrong; and the correct order will be as ${\text{ - CHO < - COOH}}$.
Hence, option (A) i.e. ${\text{RCHO > RCOR}}$ shows the correct order of priority to select a functional group.
Note: Here, in finding the highest priority of the functional group some of you may get confused between the term oxidizing agent and the oxidation because they sound similar, but they both deliver the opposite meaning. Oxidation stands for the removal of electrons and oxidizing agents stands for the accepting of electrons.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




