
The correct order of acidic nature is:
A. ${\text{HCl}}{{\text{O}}_{\text{4}}}\,{\text{ > }}\,{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}\,{\text{ > }}\,{\text{HP}}{{\text{O}}_4}$
B. ${\text{HCl}}{{\text{O}}_{\text{4}}}\,\,{\text{ > }}\,{\text{HP}}{{\text{O}}_4}{\text{ > }}\,{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}$
C. ${{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}{\text{ > }}\,{\text{HCl}}{{\text{O}}_{\text{4}}}\,{\text{ > }}\,{\text{HP}}{{\text{O}}_4}$
D. \[{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}\,{\text{ > }}\,\,{\text{HP}}{{\text{O}}_4}\,{\text{ > }}\,{\text{HCl}}{{\text{O}}_{\text{4}}}\,\]
Answer
563.4k+ views
Hint: To determine the answer we should know what are acids and the factors on which the acidic strength depends. The species which accepts electrons are known as acids. The accepting power depends upon the deficiency of electrons. So, we will determine the most electron deficient molecule.
Complete solution:
As acids accept electrons so if there any factor which increases the electrons accepting power will increase the acidic nature.
We will draw the structure of the given molecules as follows:
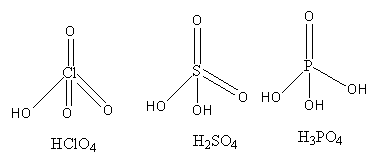
Just by looking at the structure, we cannot say anything about the acidic nature of acids so, we will compare the oxidation states. A high oxidation state means, the high positive charge on the atom means more electron-deficient central atom so it will attract the electrons easily.
High positive charge means a high effective nuclear charge of an atom so an atom having a high oxidation state will attract more electron density hence will have high acidic nature.
Now we will determine the oxidation state of the central atom of each acid.
The oxidation of chlorine atoms in \[{\text{HCl}}{{\text{O}}_{\text{4}}}\,\]is as follows:
The oxidation state of oxygen is $ - 2$ and hydrogen is $ + 1$.
$\left( { + 1} \right)\, + \,( - 2 \times 4)\, + x\, = \,0$
$x\, = \, + 7$
So, the oxidation state of chlorine is $ + 7$.
The oxidation of sulphur atoms in \[{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}\]is as follows:
The oxidation state of oxygen is $ - 2$ and hydrogen is $ + 1$.
$\left( { + 1 \times 2} \right)\, + \,( - 2 \times 4)\, + x\, = \,0$
$x\, = \, + 6$
So, the oxidation state of sulphur is$ + 6$.
The oxidation of sulphur atoms in \[{\text{HP}}{{\text{O}}_4}\]is as follows:
The oxidation state of oxygen is $ - 2$ and hydrogen is $ + 1$.
$\left( { + 1 \times 3} \right)\, + \,( - 2 \times 4)\, + x\, = \,0$
$x\, = \, + 5$
So, the oxidation state of sulphur is$ + 5$.
As we know high oxidation state means high acidity so, the correct order of acidic nature will be, ${\text{HCl}}{{\text{O}}_{\text{4}}}\,{\text{ > }}\,{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}\,{\text{ > }}\,{\text{HP}}{{\text{O}}_4}$
Therefore, option (C) is correct.
Note: According to Arrhenius and Bronsted acids are proton donors and according to Lewis acids are electron acceptors. The electrons deficient species accepts electrons so, electrons deficient species behave as acids such as aluminium trichloride which behaves as Lewis acid. Acidic nature is directly proportional to the oxidation state, effective nuclear charge, the small size of the central atom, high polarizing power of the cation, presence of an electron-withdrawing group, etc.
Complete solution:
As acids accept electrons so if there any factor which increases the electrons accepting power will increase the acidic nature.
We will draw the structure of the given molecules as follows:

Just by looking at the structure, we cannot say anything about the acidic nature of acids so, we will compare the oxidation states. A high oxidation state means, the high positive charge on the atom means more electron-deficient central atom so it will attract the electrons easily.
High positive charge means a high effective nuclear charge of an atom so an atom having a high oxidation state will attract more electron density hence will have high acidic nature.
Now we will determine the oxidation state of the central atom of each acid.
The oxidation of chlorine atoms in \[{\text{HCl}}{{\text{O}}_{\text{4}}}\,\]is as follows:
The oxidation state of oxygen is $ - 2$ and hydrogen is $ + 1$.
$\left( { + 1} \right)\, + \,( - 2 \times 4)\, + x\, = \,0$
$x\, = \, + 7$
So, the oxidation state of chlorine is $ + 7$.
The oxidation of sulphur atoms in \[{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}\]is as follows:
The oxidation state of oxygen is $ - 2$ and hydrogen is $ + 1$.
$\left( { + 1 \times 2} \right)\, + \,( - 2 \times 4)\, + x\, = \,0$
$x\, = \, + 6$
So, the oxidation state of sulphur is$ + 6$.
The oxidation of sulphur atoms in \[{\text{HP}}{{\text{O}}_4}\]is as follows:
The oxidation state of oxygen is $ - 2$ and hydrogen is $ + 1$.
$\left( { + 1 \times 3} \right)\, + \,( - 2 \times 4)\, + x\, = \,0$
$x\, = \, + 5$
So, the oxidation state of sulphur is$ + 5$.
As we know high oxidation state means high acidity so, the correct order of acidic nature will be, ${\text{HCl}}{{\text{O}}_{\text{4}}}\,{\text{ > }}\,{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}\,{\text{ > }}\,{\text{HP}}{{\text{O}}_4}$
Therefore, option (C) is correct.
Note: According to Arrhenius and Bronsted acids are proton donors and according to Lewis acids are electron acceptors. The electrons deficient species accepts electrons so, electrons deficient species behave as acids such as aluminium trichloride which behaves as Lewis acid. Acidic nature is directly proportional to the oxidation state, effective nuclear charge, the small size of the central atom, high polarizing power of the cation, presence of an electron-withdrawing group, etc.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




