
The correct IUPAC name of the following compound is:
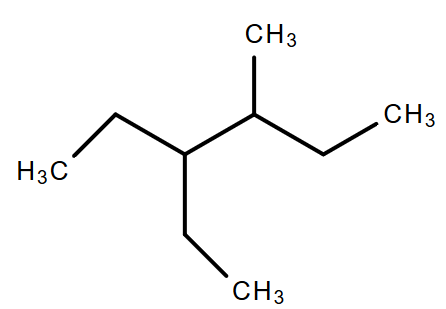
A. 4 - methyl - 3 - ethyl hexane
B. 3 - ethyl - 4 - methyl hexane
C. 3, 4 - ethyl methyl hexane
D. 4 - ethyl - 3 - methyl hexane

Answer
579.3k+ views
Hint: For this problem, we have to follow the rules of the IUPAC naming in which firstly we have to count the total number of carbon atoms which is responsible for forming the longest chain. Then we will do numbering and name the structure.
Complete step by step answer:
- In the given question, we have to explain the IUPAC name of the given molecule by following the rules of the IUPAC properly.
- Now, we know that IUPAC stands for the International Union of Pure and Applied Chemistry which is responsible for naming the compounds.
- Now, firstly we have to identify the long chain which consists of the maximum number of carbon atoms i.e.
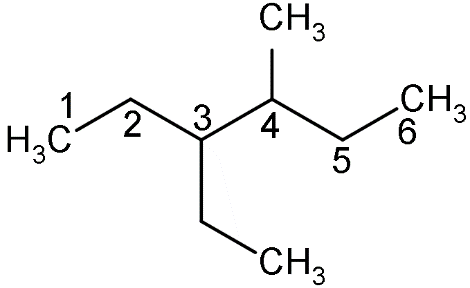
- So, we can see that the numbering of the chain starts from the left side because of the priority group.
- According to the priority order, an ethyl group is more prior group than the methyl group due to which while doing the numbering it should come first than the methyl group.
- So, if we start from right ethyl will come on 4 positions but if we start from left, ethyl will come on 3 positions.
- Now, we know that for six carbon atoms, a prefix 'hex' is used.
- Thus, the name of the compound will be 4 - ethyl - 3 - methyl hexane.
So, the correct answer is “Option D”.
Note: One should remember that the numbering will start from that point where the most prior groups first, as the priority group may differ and can be present in more than one functional group. In that case, the prefix is used for the most prior group and the suffix is used for the less prior group.
Complete step by step answer:
- In the given question, we have to explain the IUPAC name of the given molecule by following the rules of the IUPAC properly.
- Now, we know that IUPAC stands for the International Union of Pure and Applied Chemistry which is responsible for naming the compounds.
- Now, firstly we have to identify the long chain which consists of the maximum number of carbon atoms i.e.

- So, we can see that the numbering of the chain starts from the left side because of the priority group.
- According to the priority order, an ethyl group is more prior group than the methyl group due to which while doing the numbering it should come first than the methyl group.
- So, if we start from right ethyl will come on 4 positions but if we start from left, ethyl will come on 3 positions.
- Now, we know that for six carbon atoms, a prefix 'hex' is used.
- Thus, the name of the compound will be 4 - ethyl - 3 - methyl hexane.
So, the correct answer is “Option D”.
Note: One should remember that the numbering will start from that point where the most prior groups first, as the priority group may differ and can be present in more than one functional group. In that case, the prefix is used for the most prior group and the suffix is used for the less prior group.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




