
The correct IUPAC name of $[Co{{(N{{O}_{2}})}_{3}}{{(N{{H}_{3}})}_{3}}]$ is:
(a) Triammine Trinitro cobalt (III)
(b) trinitrotriammine cobalt (III)
(c) trinitrotriammine cobalt (III) ion
(d) trinitrotriammine cobaltate (III)
Answer
560.7k+ views
Hint: For writing the IUPAC name of the given coordination complex, first you should know about its IUPAC naming rule properly and then you are easily able to write its IUPAC name. now answer the given statement.
Complete step by step answer:
The IUPAC stands for the International Union of Pure and Applied Chemistry and it is the method that is used for the naming of the organic or inorganic compounds and the compounds are known by their IUPAC naming all over.
The above given compound is a coordination compound and for writing the names of the coordination compounds, there are certain rules which are as follows;
1. First of all, write the symbol of the central metal atom followed by the anionic ligands or neutral ligand and finally the cationic ligands.
2. Ligands are named alphabetically.
3. Name of the ligand is written first and then the central metal atom.
4. The naming of the ligands of some of the ligands is as;
$\begin{align}
& F'=florido\text{ } \\
& Cl'=chlorido \\
& Br'=bromido \\
& OH'=hydroxo \\
& C{{H}_{3}}COO'=acetato \\
& SC{{N}^{-}}'=thiocyanato \\
& NO_{2}^{'}=nitrito \\
& CN'=cyano \\
\end{align}$
5. To indicate the number of ligands:
In simple compounds: bi for two, tri for three, tetra for four, penta for five etc.
In complex compounds: bis for two, tris for three etc.
6. If the complex is anionic, then end the name of the metal with ate. And in case of cationic complex, there is no specific ending.
7. Oxidation state of the central metal is expressed in roman numerals.
Now considering the statement;
So, keeping in mind the above given rules for writing the IUPAC name of the coordination compound, the IUPAC name of the given complex $[Co{{(N{{O}_{2}})}_{3}}{{(N{{H}_{3}})}_{3}}]$ is as,
In this, there are two ligands i.e. $N{{O}_{2}}$ and $N{{H}_{3}}$ and Co is the central metal and oxidation state of cobalt in this is $+3$.
So, thus the IUPAC name of the complex $[Co{{(N{{O}_{2}})}_{3}}{{(N{{H}_{3}})}_{3}}]$ is Triammine Trinitro cobalt (III). The correct answer is option “A” .
Note: If bridging groups are present in the coordination compound i.e. the ligands which act as bridge between the two metal atoms, $\mu $ is written before the name of the ligand as;
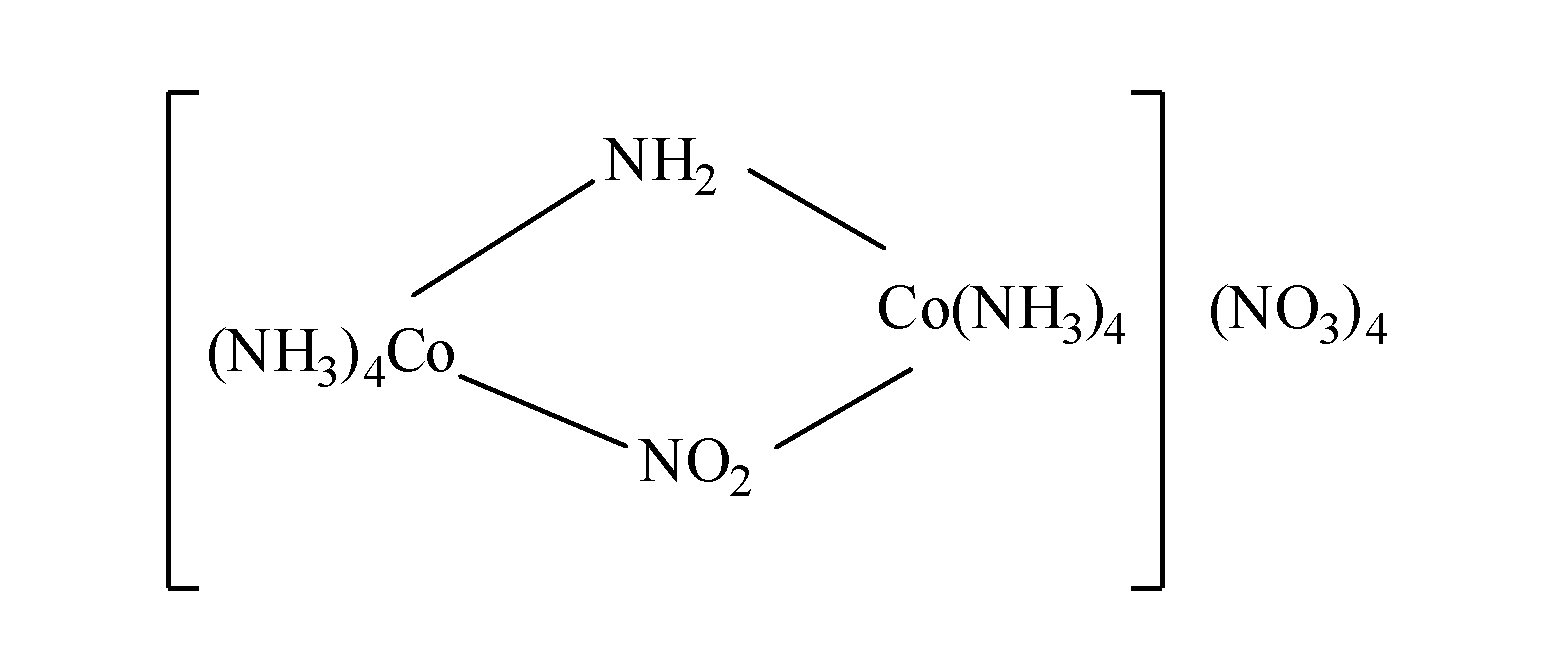
So, the IUPAC name of the complex is as: triamine cobalt(III)$\mu $-amido$\mu $-nitrito-N tetraamine cobalt (III).
Complete step by step answer:
The IUPAC stands for the International Union of Pure and Applied Chemistry and it is the method that is used for the naming of the organic or inorganic compounds and the compounds are known by their IUPAC naming all over.
The above given compound is a coordination compound and for writing the names of the coordination compounds, there are certain rules which are as follows;
1. First of all, write the symbol of the central metal atom followed by the anionic ligands or neutral ligand and finally the cationic ligands.
2. Ligands are named alphabetically.
3. Name of the ligand is written first and then the central metal atom.
4. The naming of the ligands of some of the ligands is as;
$\begin{align}
& F'=florido\text{ } \\
& Cl'=chlorido \\
& Br'=bromido \\
& OH'=hydroxo \\
& C{{H}_{3}}COO'=acetato \\
& SC{{N}^{-}}'=thiocyanato \\
& NO_{2}^{'}=nitrito \\
& CN'=cyano \\
\end{align}$
5. To indicate the number of ligands:
In simple compounds: bi for two, tri for three, tetra for four, penta for five etc.
In complex compounds: bis for two, tris for three etc.
6. If the complex is anionic, then end the name of the metal with ate. And in case of cationic complex, there is no specific ending.
7. Oxidation state of the central metal is expressed in roman numerals.
Now considering the statement;
So, keeping in mind the above given rules for writing the IUPAC name of the coordination compound, the IUPAC name of the given complex $[Co{{(N{{O}_{2}})}_{3}}{{(N{{H}_{3}})}_{3}}]$ is as,
In this, there are two ligands i.e. $N{{O}_{2}}$ and $N{{H}_{3}}$ and Co is the central metal and oxidation state of cobalt in this is $+3$.
So, thus the IUPAC name of the complex $[Co{{(N{{O}_{2}})}_{3}}{{(N{{H}_{3}})}_{3}}]$ is Triammine Trinitro cobalt (III). The correct answer is option “A” .
Note: If bridging groups are present in the coordination compound i.e. the ligands which act as bridge between the two metal atoms, $\mu $ is written before the name of the ligand as;

So, the IUPAC name of the complex is as: triamine cobalt(III)$\mu $-amido$\mu $-nitrito-N tetraamine cobalt (III).
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




