
The correct increasing order of trans-effect of the following species is:
A. $B{r^ - } > C{N^ - } > N{H_3} > {C_6}H_5^ - $
B. $C{N^ - } > B{r^ - } > {C_6}H_5^ - > N{H_3}$
C. $N{H_3} > C{N^ - } > B{r^ - } > {C_6}H_5^ - $
D. $C{N^ - } > {C_6}H_5^ - > B{r^ - } > N{H_3}$
Answer
569.7k+ views
Hint: We have to remember that the Trans effect also called as kinetic Tran’s effect is the effect due to which a ligand can substitute another ligand which is Trans to it. We know that in many compounds the ligands exist in cis position and in trans position.
Complete step by step answer:
Let us take an example to understand the cis and trans effect.
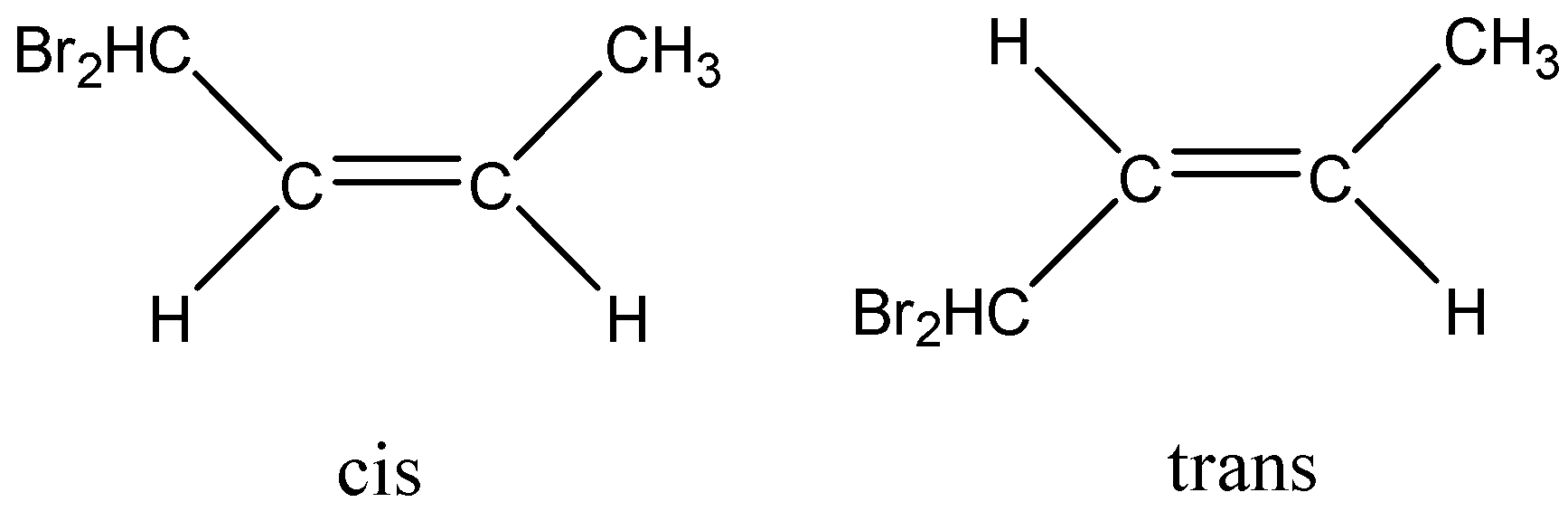
In the above example, we have cis-trans isomers. In the first one the alkyl halide group is on the side of the alkyl group attached to the parent carbon atom chain. This orientation is called cis position. While in the second structure the alkyl halide group is on the opposite side of the alkyl group attached. Hence, this orientation is called trans position. The trans effect can vary for different ligands. Stronger the Trans effect the more is the yield of the Trans compound in majority.
Trans effect is more for the ligands which are trans directing. The general and practically observed ascending order of the ligand mentioned above can be given as:
\[
{F^ - } < H{O^ - } < {H_2}O < N{H_3} < py < C{l^ - } < B{r^ - } < {I^ - } \\
< SC{N^ - } < NO_2^ - < SC{\left( {N{H_3}} \right)_2} < P{h^ - } < SO_2^ - < P{R_3} \\
< As{R_3} < S{R_2} < C{H_3} < {H^ - } < NO < CO < N{C^ - } < {C_2}{H_4} \\
\]
Where $N{H_3}$ is the least Trans directing and $C{N^ - }$ is strongly Trans directing.
So, the correct answer is Option A.
Note: We have to remember that for ligands, their electronic properties determine the trans or cis effect in the orientation. Trans and cis effect generally exist in square planar geometry. Lesser the electronegativity the higher is the trans directing effect of the ligand. Strong trans effect can also be viewed in terms of strong sigma bond donor and strong pi bond acceptor. Cis and trans are basically the isomers of each other. In reactions, both the cis and trans isomers are yielded. But the majority product depends on the Trans directing and cis directing property of the ligand.
Complete step by step answer:
Let us take an example to understand the cis and trans effect.

In the above example, we have cis-trans isomers. In the first one the alkyl halide group is on the side of the alkyl group attached to the parent carbon atom chain. This orientation is called cis position. While in the second structure the alkyl halide group is on the opposite side of the alkyl group attached. Hence, this orientation is called trans position. The trans effect can vary for different ligands. Stronger the Trans effect the more is the yield of the Trans compound in majority.
Trans effect is more for the ligands which are trans directing. The general and practically observed ascending order of the ligand mentioned above can be given as:
\[
{F^ - } < H{O^ - } < {H_2}O < N{H_3} < py < C{l^ - } < B{r^ - } < {I^ - } \\
< SC{N^ - } < NO_2^ - < SC{\left( {N{H_3}} \right)_2} < P{h^ - } < SO_2^ - < P{R_3} \\
< As{R_3} < S{R_2} < C{H_3} < {H^ - } < NO < CO < N{C^ - } < {C_2}{H_4} \\
\]
Where $N{H_3}$ is the least Trans directing and $C{N^ - }$ is strongly Trans directing.
So, the correct answer is Option A.
Note: We have to remember that for ligands, their electronic properties determine the trans or cis effect in the orientation. Trans and cis effect generally exist in square planar geometry. Lesser the electronegativity the higher is the trans directing effect of the ligand. Strong trans effect can also be viewed in terms of strong sigma bond donor and strong pi bond acceptor. Cis and trans are basically the isomers of each other. In reactions, both the cis and trans isomers are yielded. But the majority product depends on the Trans directing and cis directing property of the ligand.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




