
The correct experiment set-up for separating a substance by sublimation is:
(A)
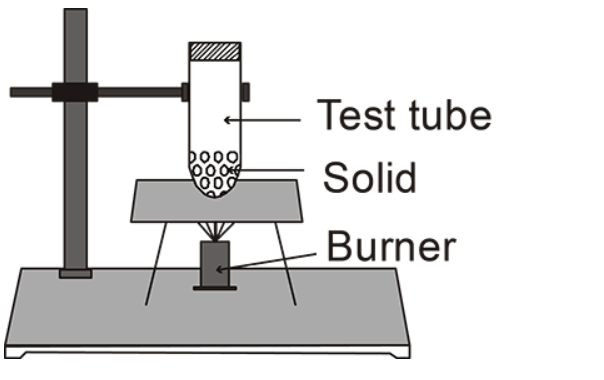
(B)
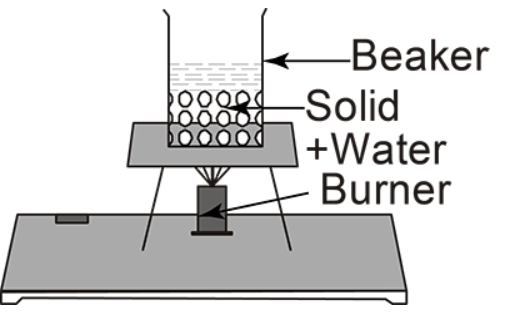
(C)
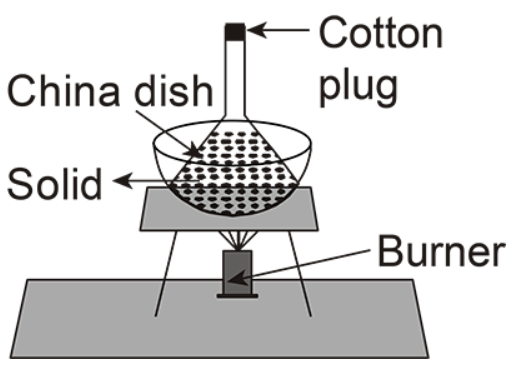
(D)
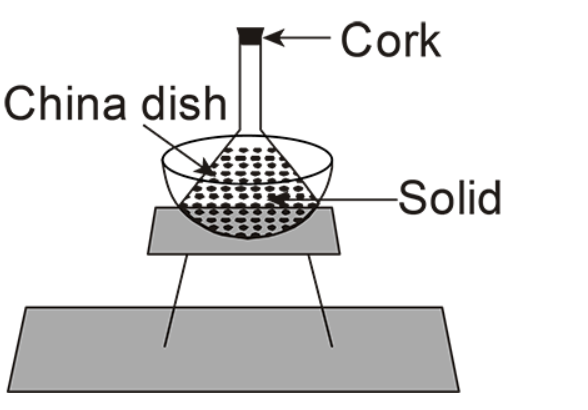




Answer
537.6k+ views
Hint :In the given options, C is the correct option.
So in order to separate a mixture from non-volatile compound to volatile one, sublimation process is used.
The mixture is first heated with the help of a burner. The solid compound sublimes after heating and turns into a gaseous state. Then the gas passes out from the cotton plug.
Complete Step By Step Answer:
In the given options, C is the correct option.
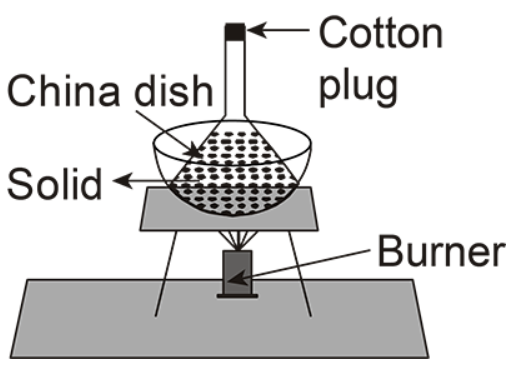
In this setup there is a base in which their burner is placed and a sheet is present at the base of the china dish, the solid is placed inside the funnel and the cotton plug is placed at the top of it.
Here, sublimation is generally a process in which the solid state phase is changed into gaseous state without converting it into a liquid form.
The mixture is first heated with the help of a burner. The solid compound sublimes after heating and turns into a gaseous state. Then the gas passes out from the cotton plug.
Note :
Sublimation is a process in which matter undergoes direct phase transition from a solid state to gas. This won’t involve the liquid transition state. For example we know that ammonium chloride is the perfect example of changing a solid to directly in tis gaseous form on constant heating. So in order to separate a mixture from non-volatile compound to volatile one, sublimation process is used.
So in order to separate a mixture from non-volatile compound to volatile one, sublimation process is used.
The mixture is first heated with the help of a burner. The solid compound sublimes after heating and turns into a gaseous state. Then the gas passes out from the cotton plug.
Complete Step By Step Answer:
In the given options, C is the correct option.

In this setup there is a base in which their burner is placed and a sheet is present at the base of the china dish, the solid is placed inside the funnel and the cotton plug is placed at the top of it.
Here, sublimation is generally a process in which the solid state phase is changed into gaseous state without converting it into a liquid form.
The mixture is first heated with the help of a burner. The solid compound sublimes after heating and turns into a gaseous state. Then the gas passes out from the cotton plug.
Note :
Sublimation is a process in which matter undergoes direct phase transition from a solid state to gas. This won’t involve the liquid transition state. For example we know that ammonium chloride is the perfect example of changing a solid to directly in tis gaseous form on constant heating. So in order to separate a mixture from non-volatile compound to volatile one, sublimation process is used.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




