
The correct chemical formula of nitrous oxide is
a.) \[{{N}_{2}}O\]
b.) NO
c.) \[N{{O}_{2\,}}\]
d.) \[{{N}_{2}}{{O}_{3}}\]
Answer
600.6k+ views
Hint: A chemical formula is the presentation of the chemical proportions of the constituent atoms that constitute a particular molecule or a compound. Some of the non-metal exist as simple molecules with two atoms joined together. These elements are diatomic.
Complete step by step solution:
Nitrous Oxide is also known as laughing gas. It is a chemical compound or an oxide of nitrogen with the chemical formula \[{{N}_{2}}O\]. It is non-flammable at room temperature with a slight metallic taste and smell.
Nitrous Oxide is prepared at industrial scale by heating ammonium nitrate at about 250℃.
The chemical reaction for it.
\[N{{H}_{4}}N{{O}_{3}}\to \,2{{H}_{2}}O\,+\,{{N}_{2}}O\]
The molecular mass of nitrous oxide is 44g/mol and molecular shape is linear.
When we draw the Lewis structure of nitrous oxide it has a total of 16 valence electrons (5 from each of the nitrogen and 6 from oxygen). Lewis structure for nitrous oxide can be drawn in two forms.
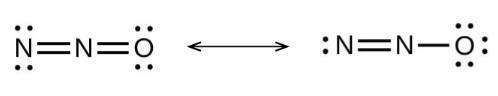
Let’s know why other options are not correct.
a) NO is a binary compound of nitrogen and oxygen, it is called nitric oxide. Its anions and cations are nitroxide (\[N{{O}^{-}}\]) and nitronium ion (\[N{{O}^{+}}\]).
b) \[N{{O}_{2\,}}\] is called nitrogen dioxide, which is prepared by the oxidation of nitric oxide.
c) \[{{N}_{2}}{{O}_{3}}\] is called dinitrogen trioxide, it forms upon by equal mixing of nitric oxide and nitrogen dioxide.
So, from the above statements we can conclude that the chemical formula for nitrous oxide is \[{{N}_{2}}O\]. Therefore, the correct option is (a).
Note: IUPAC name of Nitrous oxide is Dinitrogen monoxide. It is also commonly known as laughing gas. It is sweet-smelling, colourless and nonflammable at room temperature. Nitrous oxide is heavier than air.
Complete step by step solution:
Nitrous Oxide is also known as laughing gas. It is a chemical compound or an oxide of nitrogen with the chemical formula \[{{N}_{2}}O\]. It is non-flammable at room temperature with a slight metallic taste and smell.
Nitrous Oxide is prepared at industrial scale by heating ammonium nitrate at about 250℃.
The chemical reaction for it.
\[N{{H}_{4}}N{{O}_{3}}\to \,2{{H}_{2}}O\,+\,{{N}_{2}}O\]
The molecular mass of nitrous oxide is 44g/mol and molecular shape is linear.
When we draw the Lewis structure of nitrous oxide it has a total of 16 valence electrons (5 from each of the nitrogen and 6 from oxygen). Lewis structure for nitrous oxide can be drawn in two forms.

Let’s know why other options are not correct.
a) NO is a binary compound of nitrogen and oxygen, it is called nitric oxide. Its anions and cations are nitroxide (\[N{{O}^{-}}\]) and nitronium ion (\[N{{O}^{+}}\]).
b) \[N{{O}_{2\,}}\] is called nitrogen dioxide, which is prepared by the oxidation of nitric oxide.
c) \[{{N}_{2}}{{O}_{3}}\] is called dinitrogen trioxide, it forms upon by equal mixing of nitric oxide and nitrogen dioxide.
So, from the above statements we can conclude that the chemical formula for nitrous oxide is \[{{N}_{2}}O\]. Therefore, the correct option is (a).
Note: IUPAC name of Nitrous oxide is Dinitrogen monoxide. It is also commonly known as laughing gas. It is sweet-smelling, colourless and nonflammable at room temperature. Nitrous oxide is heavier than air.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




