
The correct bond order in which the ${\text{O}} - {\text{O}}$ bond length increases as:
A. ${{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}\,{\text{ < }}\,{{\text{O}}_{\text{2}}}{\text{ < }}\,{{\text{O}}_{\text{3}}}$
B. ${{\text{O}}_{\text{3}}}{\text{ < }}\,\,{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}\,{\text{ < }}\,{{\text{O}}_{\text{2}}}$
C. ${{\text{O}}_{\text{2}}}\,{\text{ < }}\,{{\text{O}}_{\text{3}}}{\text{ < }}\,\,{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}$
D. ${{\text{O}}_2}{\text{ < }}\,\,{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}\,{\text{ < }}\,{{\text{O}}_3}$
Answer
578.1k+ views
Hint: Bond length is the distance between the bonded atoms. Bond length depends upon the bond strength and bond order. As the bond strength or bond
order increases the bond length decreases.
Complete step by step answer:
The bond length depends upon the bond strength which in turn depends upon the bond order. As the bond order increases the bond strength increases which in turn decreases the bond length.
So, the relations in bond length, bond order, and bond strength are as follows:
${\text{B}}{\text{.L}}{\text{.}} \propto \dfrac{1}{{{\text{B}}{\text{.O}}{\text{.}}}} \propto \dfrac{1}{{{\text{B}}{\text{.S}}{\text{.}}}}$
Where, B.L. is the bond length, B.O. is the bond order and B.S. is the bond strength.
The structures of all three molecules are as follows:
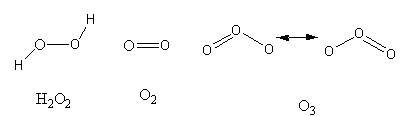
The ${\text{O}} - {\text{O}}$ chemical bonds in ${{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}$ is one, in ${{\text{O}}_2}$ is two and in ${{\text{O}}_3}$ is in-between one and two because in ${{\text{O}}_3}$ some resonating structures are possible which are interconvertible.
So, the decreasing order of bond order or bond strength is,
${{\text{O}}_2}\, > \,{{\text{O}}_3}\, > \,{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}$
Bond order is inversely proportional to bond length so, the increasing order of bond length is,
${{\text{O}}_{\text{2}}}\,{\text{ < }}\,{{\text{O}}_{\text{3}}}{\text{ < }}\,\,{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}$
Therefore, option (C) ${{\text{O}}_{\text{2}}}\,{\text{ < }}\,{{\text{O}}_{\text{3}}}{\text{ < }}\,\,{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}$ , is correct.
Note: Bond order is defined as the number of chemical bonds between a pair of atoms. Bond length is inversely proportional to the bond strength and bond order. Bond strength is directly proportional to the bond order. If the structure is known then the bond order is determined by the molecular orbitals diagram. The formula used to determine the bond order is as follows: $\dfrac{{{\text{bonding}}\,{\text{electron}}\,\, - \,\,{\text{antibonding electron}}}}{2}$ .
order increases the bond length decreases.
Complete step by step answer:
The bond length depends upon the bond strength which in turn depends upon the bond order. As the bond order increases the bond strength increases which in turn decreases the bond length.
So, the relations in bond length, bond order, and bond strength are as follows:
${\text{B}}{\text{.L}}{\text{.}} \propto \dfrac{1}{{{\text{B}}{\text{.O}}{\text{.}}}} \propto \dfrac{1}{{{\text{B}}{\text{.S}}{\text{.}}}}$
Where, B.L. is the bond length, B.O. is the bond order and B.S. is the bond strength.
The structures of all three molecules are as follows:

The ${\text{O}} - {\text{O}}$ chemical bonds in ${{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}$ is one, in ${{\text{O}}_2}$ is two and in ${{\text{O}}_3}$ is in-between one and two because in ${{\text{O}}_3}$ some resonating structures are possible which are interconvertible.
So, the decreasing order of bond order or bond strength is,
${{\text{O}}_2}\, > \,{{\text{O}}_3}\, > \,{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}$
Bond order is inversely proportional to bond length so, the increasing order of bond length is,
${{\text{O}}_{\text{2}}}\,{\text{ < }}\,{{\text{O}}_{\text{3}}}{\text{ < }}\,\,{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}$
Therefore, option (C) ${{\text{O}}_{\text{2}}}\,{\text{ < }}\,{{\text{O}}_{\text{3}}}{\text{ < }}\,\,{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}$ , is correct.
Note: Bond order is defined as the number of chemical bonds between a pair of atoms. Bond length is inversely proportional to the bond strength and bond order. Bond strength is directly proportional to the bond order. If the structure is known then the bond order is determined by the molecular orbitals diagram. The formula used to determine the bond order is as follows: $\dfrac{{{\text{bonding}}\,{\text{electron}}\,\, - \,\,{\text{antibonding electron}}}}{2}$ .
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




