
The coordination number of Al in the crystalline state of $AlC{{l}_{3}}$ is:
Answer
569.4k+ views
Hint: The number of atoms surrounded around the metal atom is called coordination number. Coordination number of some metals is going to change with the physical state of the compound. The coordination number of the central metal in liquid state is different from the coordination of the central metal atom in solid state.
Complete Solution :
- In the question it is given the coordination number of aluminium in the crystalline state of aluminum chloride.
- We have to know the structure of the crystalline aluminium chloride to know about the coordination number of aluminum.
- The structure of the aluminum chloride in liquid state is different from the structure of the aluminium chloride in crystalline state.
- The structure of the aluminium chloride in liquid state is as follows.
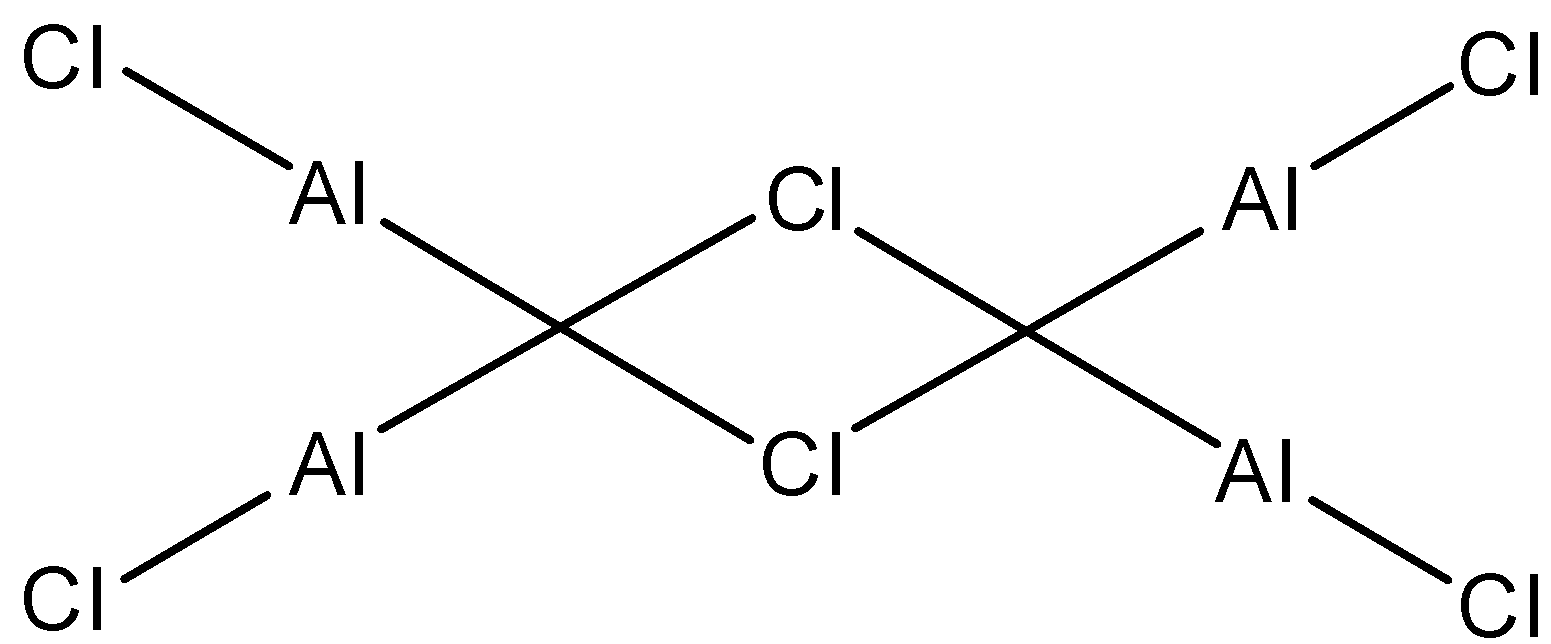
- In liquid state aluminium chloride exists as dimer and from the above structure the coordination number of aluminium is 4 in aluminium chloride.
- Coming to the structure of the aluminium chloride in crystalline state and it as follows.

- From the above structure we can say that the coordination number of aluminium in aluminium chloride is 6.
- Therefore the coordination of aluminium in crystalline aluminium chloride is 6.
Note: In solid state or crystalline state the chloride ions form a space lattice with aluminium ions by occupying the octahedral voids. Therefore the coordination number of aluminium in crystalline aluminium chloride is 6.
Complete Solution :
- In the question it is given the coordination number of aluminium in the crystalline state of aluminum chloride.
- We have to know the structure of the crystalline aluminium chloride to know about the coordination number of aluminum.
- The structure of the aluminum chloride in liquid state is different from the structure of the aluminium chloride in crystalline state.
- The structure of the aluminium chloride in liquid state is as follows.

- In liquid state aluminium chloride exists as dimer and from the above structure the coordination number of aluminium is 4 in aluminium chloride.
- Coming to the structure of the aluminium chloride in crystalline state and it as follows.

- From the above structure we can say that the coordination number of aluminium in aluminium chloride is 6.
- Therefore the coordination of aluminium in crystalline aluminium chloride is 6.
Note: In solid state or crystalline state the chloride ions form a space lattice with aluminium ions by occupying the octahedral voids. Therefore the coordination number of aluminium in crystalline aluminium chloride is 6.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




