
The compounds A, B and C in the following reaction sequence are
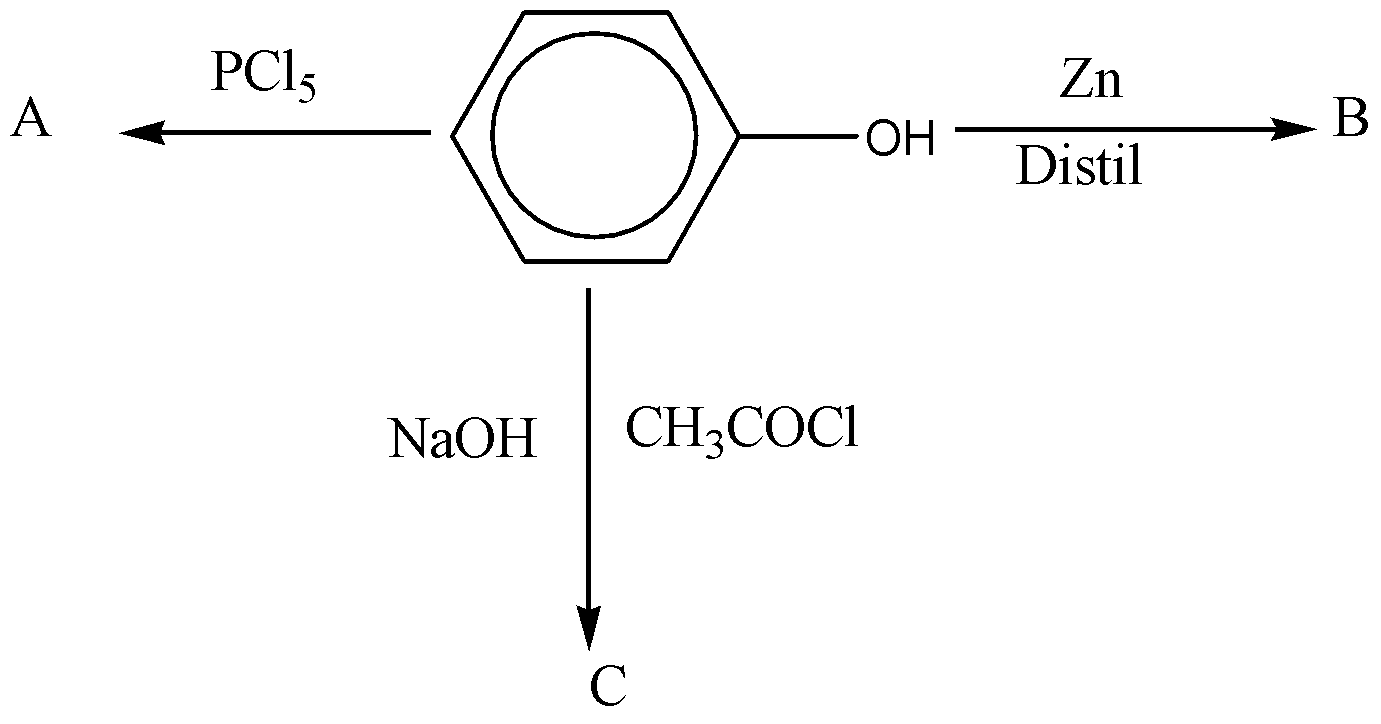
A. Chlorobenzene, benzene, methyl benzoate
B. Triphenyl phosphate, benzene, phenyl acetate
C. Benzyl chloride, benzene, phenyl acetate
D. Benzyl chloride, benzene, phenylacetyl chloride

Answer
572.1k+ views
Hint: When Phenol reacts with Phosphorus pentachloride, triphenyl phosphate is formed. When Phenol reacts with Zinc dust, there is a reduction reaction of phenol with zinc dust. When phenol is reacted with acetyl chloride in the presence of sodium hydroxide, it leads to the formation of phenyl esters.
Complete step by step answer:
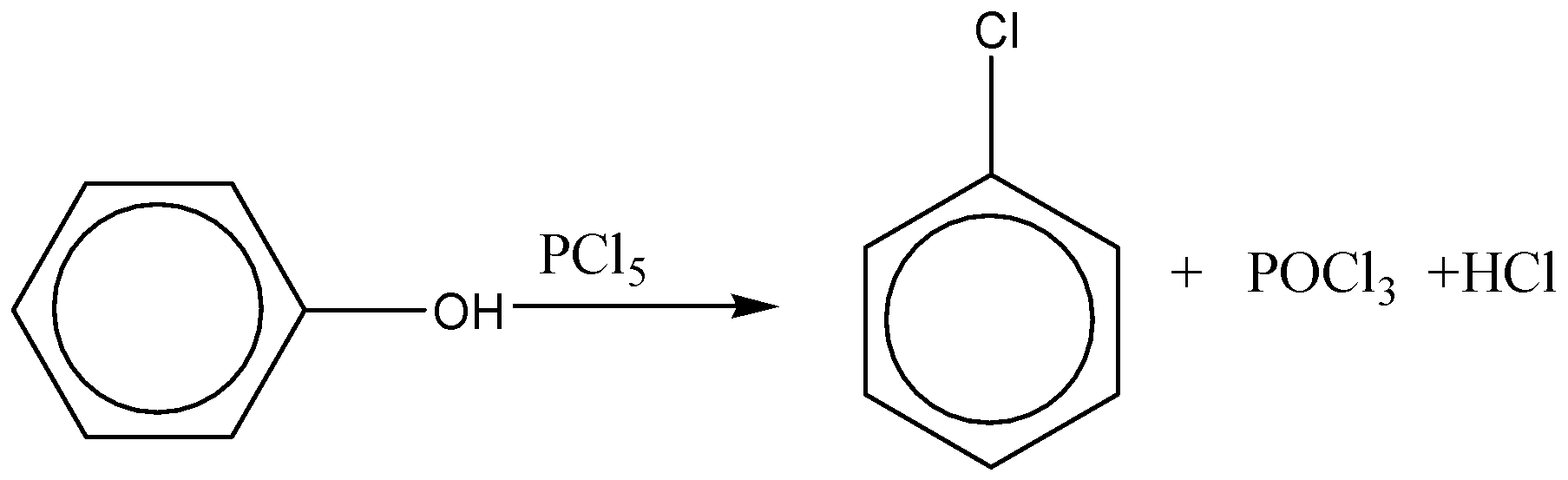
Phenyl reacts with \[PC{l_5}\] to give only a small amount of chlorobenzene, the main product is triphenyl phosphate, ${({C_6}{H_5})_3}P{O_4}$, the ester of phosphoric acid. With \[PC{l_3}\], we get ester of phosphoric acid, ${({C_6}{H_5})_3}P{O_3}$.
However, the yield of chlorobenzene is very small and the main product is triphenyl phosphate, which is mainly formed by the reaction between phenol and \[PC{l_3}\]
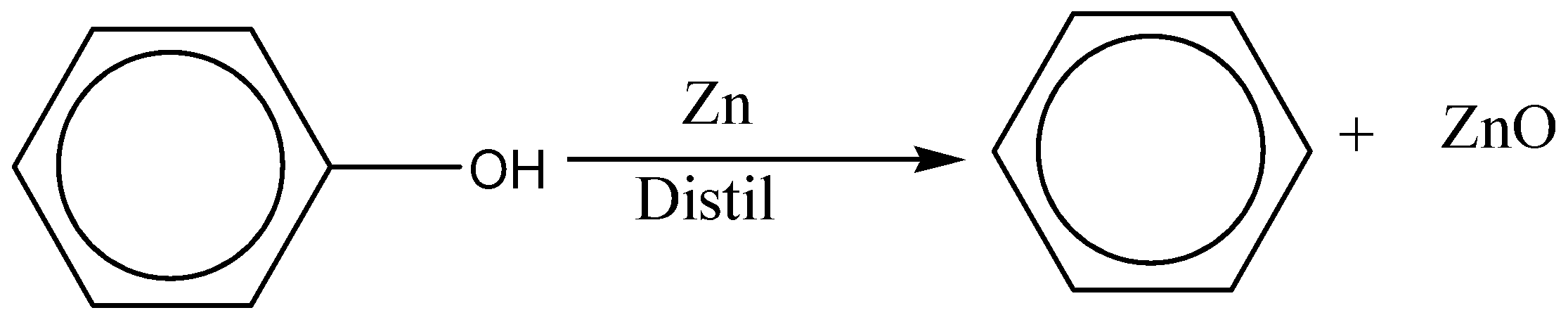
There is a reduction reaction of phenol with zinc dust, the product formed are benzene and ZnO. The reaction can be given as follow:
${C_6}{H_5}OH + Zn \to {C_6}{H_6} + ZnO$
When phenol is reacted with acetyl chloride in the presence of sodium hydroxide, it leads to the formation of phenyl esters and this reaction will lead to the formation of phenyl ethanoate. This reaction shows the acidic nature of phenol.
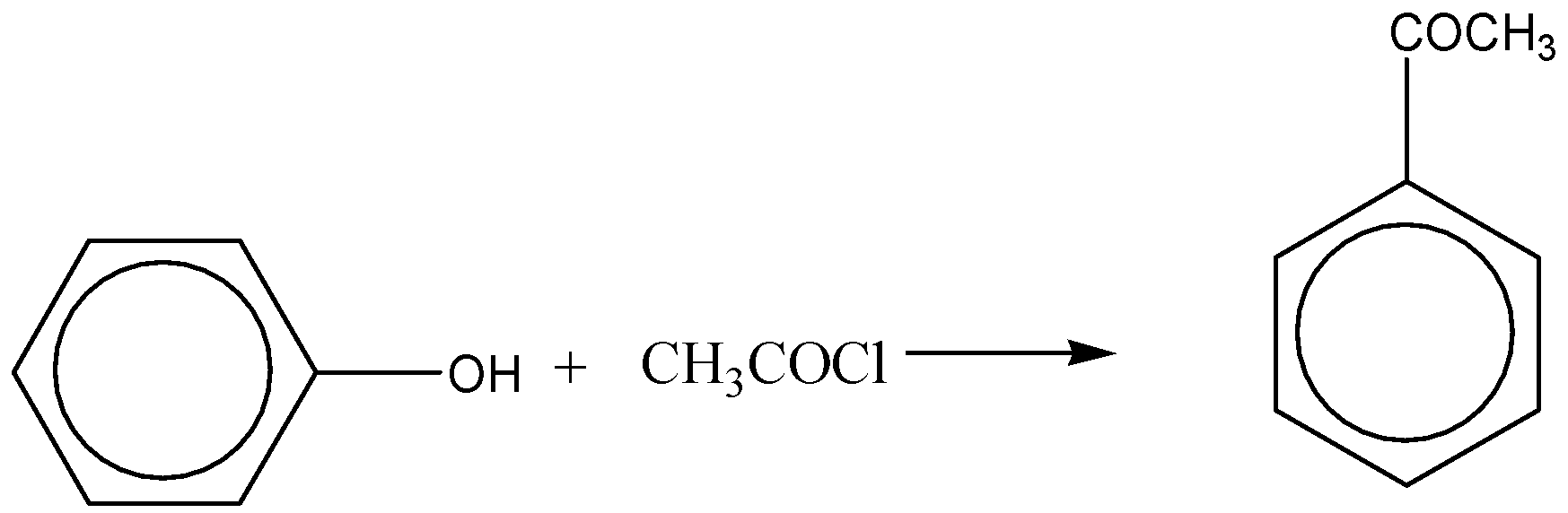
Hence, the compounds A, B and C in the above reaction sequence are Triphenyl phosphate, benzene, phenyl acetate respectively.
Therefore, the correct answer is option (B).
Note: The yield of the reaction in phenol with zinc dust for the formation of benzene is lower. Homolytic fission is the splitting of the pair of electrons between two separated atoms. It is a reduction reaction of phenol to benzene by the zinc dust. So, zinc is the reducing agent. Benzene formed is volatile in nature and it can be separated by fractional distillation.
Complete step by step answer:
Phenyl reacts with \[PC{l_5}\] to give only a small amount of chlorobenzene, the main product is triphenyl phosphate, ${({C_6}{H_5})_3}P{O_4}$, the ester of phosphoric acid. With \[PC{l_3}\], we get ester of phosphoric acid, ${({C_6}{H_5})_3}P{O_3}$.
However, the yield of chlorobenzene is very small and the main product is triphenyl phosphate, which is mainly formed by the reaction between phenol and \[PC{l_3}\]

There is a reduction reaction of phenol with zinc dust, the product formed are benzene and ZnO. The reaction can be given as follow:
${C_6}{H_5}OH + Zn \to {C_6}{H_6} + ZnO$

When phenol is reacted with acetyl chloride in the presence of sodium hydroxide, it leads to the formation of phenyl esters and this reaction will lead to the formation of phenyl ethanoate. This reaction shows the acidic nature of phenol.
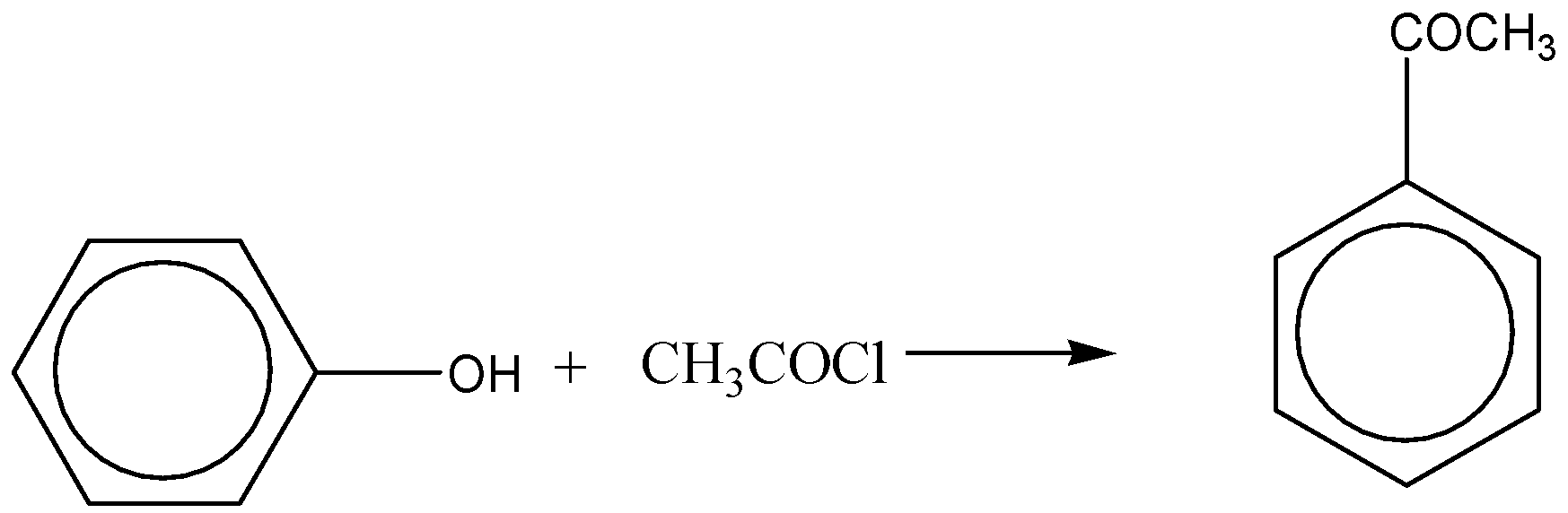
Hence, the compounds A, B and C in the above reaction sequence are Triphenyl phosphate, benzene, phenyl acetate respectively.
Therefore, the correct answer is option (B).
Note: The yield of the reaction in phenol with zinc dust for the formation of benzene is lower. Homolytic fission is the splitting of the pair of electrons between two separated atoms. It is a reduction reaction of phenol to benzene by the zinc dust. So, zinc is the reducing agent. Benzene formed is volatile in nature and it can be separated by fractional distillation.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




