
The compound that will have a permanent dipole moment among the following is:
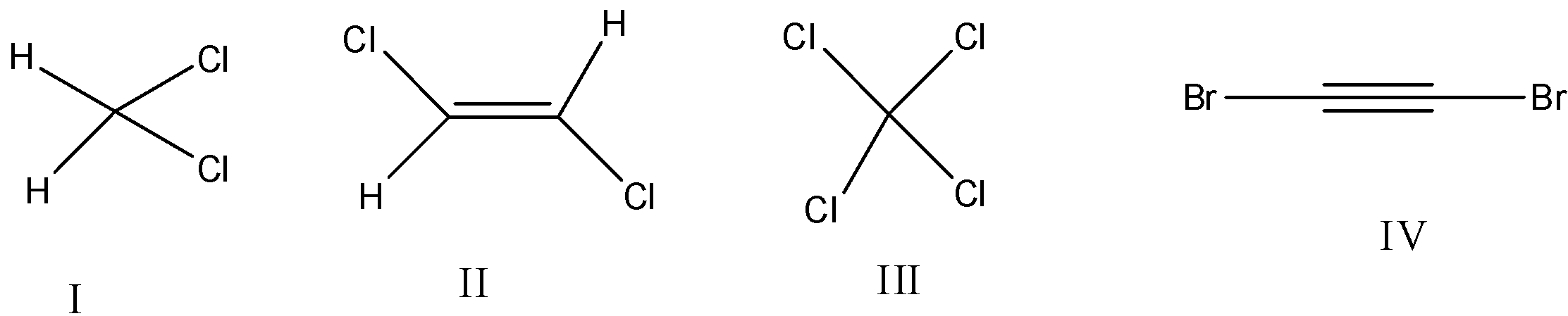
A. I
B. II
C. III
D. IV

Answer
522.9k+ views
Hint: The dipole moment is going to arise in the covalent compounds due to the difference in the electronegative values of the atoms present in the molecule. For symmetrical molecules the net dipole moment will be zero.
Complete answer:
- In the question it is asked to find the molecule among the given structures which should contain a permanent dipole moment.
- Dipole moment is nothing but the charge separation of the atom in the molecule.
- If the electrons are going to be attracted by the atoms equally in both the directions (means opposite direction) then the net dipole moment will be zero for the particular molecule.
- Coming to the given structures.
- Structure I,
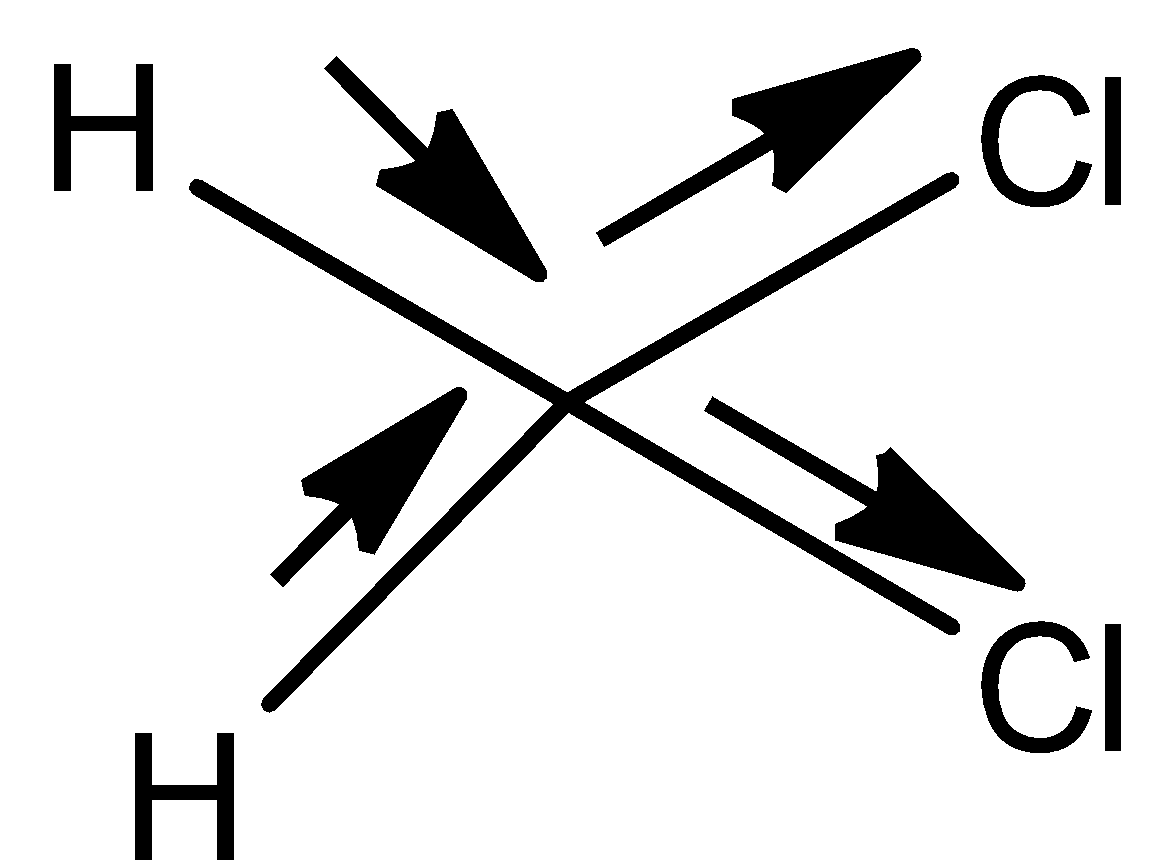
- In the above structure we can see that the electrons only move in one direction that too towards chlorine because of the higher electronegativity of the chlorine.
- Then the permanent dipole moment of the molecule will not be zero.
- Coming to structure II,
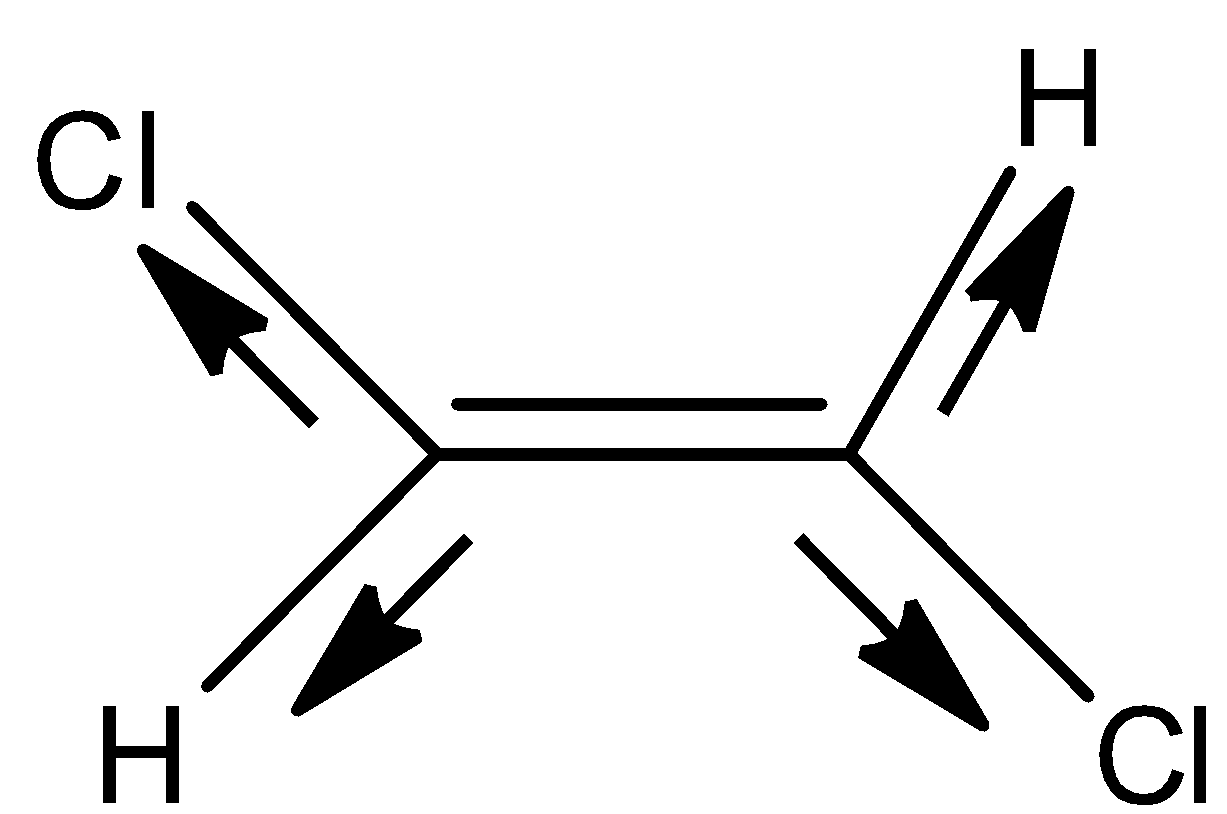
- In the above structure we can see that the electrons move equally in the opposite direction due to the presence of the same type of atoms with same electronegativity.
- Therefore the net dipole moment of the molecule is going to be zero.
- Coming to structure III,
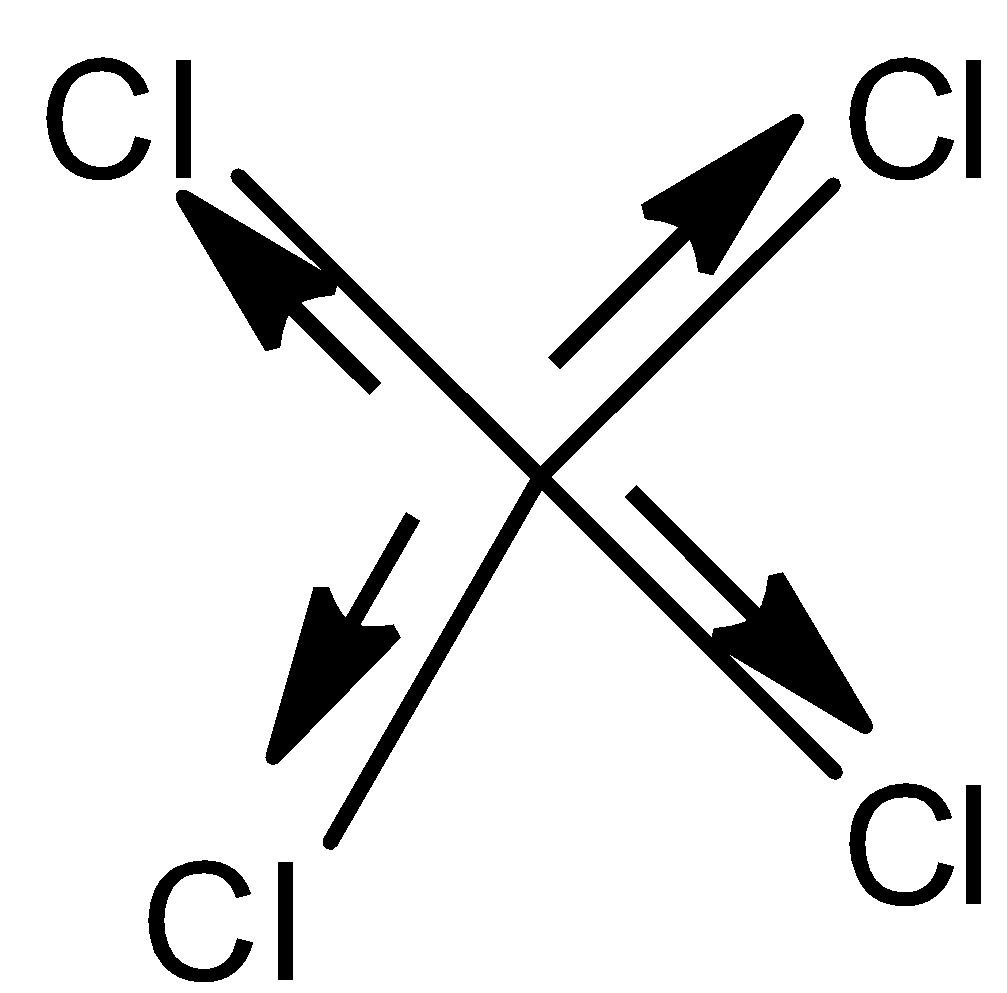
- In the above structure we can see that the electrons move equally in opposite direction due to the presence of the same type of atoms with the same electronegativity.
- Therefore the net dipole moment of the molecule is going to be zero.
- Coming to structure IV,
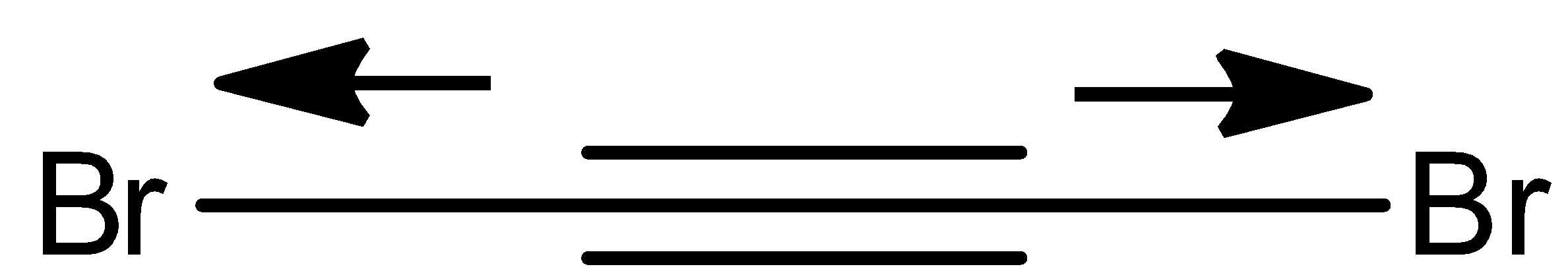
In the above structure we can see that the electrons move equally in opposite direction due to the presence of the same type of atoms with the same electronegativity.
- Therefore the net dipole moment of the molecule is going to be zero.
- Therefore the molecule which does not have the net dipole moment among the given structures is I.
So, the correct option is A.
Note:
The highly electronegative atoms should not present in the opposite direction in the molecule. If the high electronegativity value containing atoms are present in the opposite direction then the net dipole moment is going to be zero.
Complete answer:
- In the question it is asked to find the molecule among the given structures which should contain a permanent dipole moment.
- Dipole moment is nothing but the charge separation of the atom in the molecule.
- If the electrons are going to be attracted by the atoms equally in both the directions (means opposite direction) then the net dipole moment will be zero for the particular molecule.
- Coming to the given structures.
- Structure I,

- In the above structure we can see that the electrons only move in one direction that too towards chlorine because of the higher electronegativity of the chlorine.
- Then the permanent dipole moment of the molecule will not be zero.
- Coming to structure II,

- In the above structure we can see that the electrons move equally in the opposite direction due to the presence of the same type of atoms with same electronegativity.
- Therefore the net dipole moment of the molecule is going to be zero.
- Coming to structure III,

- In the above structure we can see that the electrons move equally in opposite direction due to the presence of the same type of atoms with the same electronegativity.
- Therefore the net dipole moment of the molecule is going to be zero.
- Coming to structure IV,
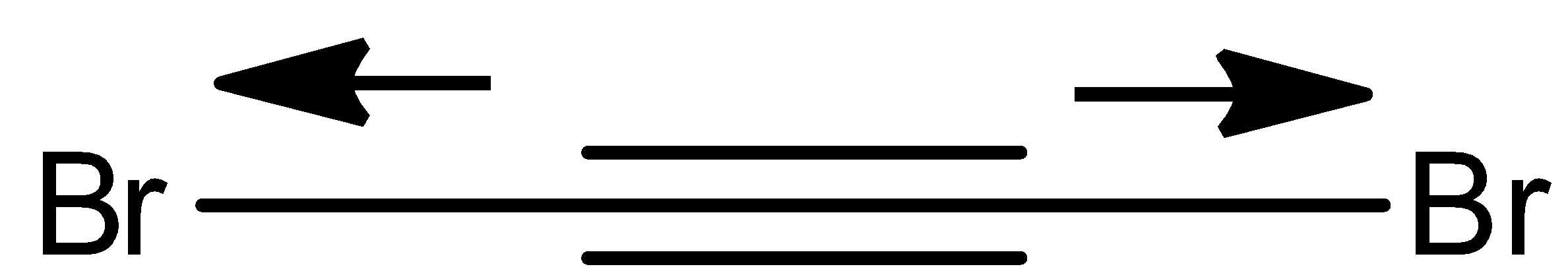
In the above structure we can see that the electrons move equally in opposite direction due to the presence of the same type of atoms with the same electronegativity.
- Therefore the net dipole moment of the molecule is going to be zero.
- Therefore the molecule which does not have the net dipole moment among the given structures is I.
So, the correct option is A.
Note:
The highly electronegative atoms should not present in the opposite direction in the molecule. If the high electronegativity value containing atoms are present in the opposite direction then the net dipole moment is going to be zero.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




