
The compound on reaction with $ NaI{O_4} $ in the presence of $ \;KMn{O_4} $ gives
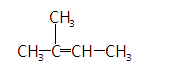
A) $ C{H_3}COC{H_3} $
B) $ C{H_3}COC{H_3} + C{H_3}COOH\;\;\;\; $
C) $ C{H_3}COC{H_3} + C{H_3}CHO\; $
D) $ C{H_3}CHO + C{O_2} $
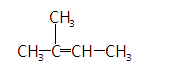
Answer
537.6k+ views
Hint :Here the reactant is alkene which reacts with Lemieux reagents. $ \;KMn{O_4} $ is a strong oxidizing agent which oxidizes alkene to cis-diol and $ NaI{O_4} $ cleaves the bond between two carbon atoms of the obtained product.
Complete Step By Step Answer:
The given molecular formula is- $ C{H_3} - C\left( {C{H_3}} \right) = CH - C{H_3} $
It is also called is isopentane and its IUPAC name is $ 3 - methylbut - 2 - ene $
This compound reacts with given reagents- sodium per-iodate and potassium permanganate. . The aqueous solution of both the reagent is known as Lemieux reagent
We have to find the product.
Here, $ \;KMn{O_4} $ is oxidizing agent so it oxidizes the alkene and cis-diol product is obtained. The reaction is given as-
$ C{H_3} - C\left( {C{H_3}} \right) = CH - C{H_3}\xrightarrow{{KMn{O_4}}}C{H_3} - C\left( {C{H_3}} \right)\left( {OH} \right) - CH\left( {OH} \right) - C{H_3} $
This obtained product is then cleaved by sodium periodate into aldehyde and ketone. The reaction is given as-
$ C{H_3} - C\left( {C{H_3}} \right)\left( {OH} \right) - CH\left( {OH} \right) - C{H_3}\xrightarrow[{ - {H_2}O}]{{NaI{O_4}}}C{H_3}COC{H_3} + C{H_3}CHO $
Now, since aldehyde is more reactive than ketone it is further oxidized by $ KMn{O_4} $ to carboxylic acid. The reaction is given as-
$ C{H_3}CHO\xrightarrow{{KMn{O_4}}}C{H_3}COOH $
The whole reaction can be summarized as-
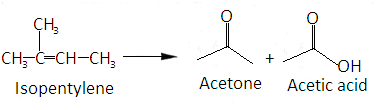
Hence correct answer is option B.
Note :
Students may go wrong if they think that the reaction stops when aldehyde is formed and choose the option C which is incorrect.
We know that $ KMn{O_4} $ is a strong oxidizing agent which can oxidize the reagent in any medium whether it is acidic, basic or neutral so it further oxidizes aldehyde to carboxylic acid. $ KMn{O_4} $ Has following properties-
It is odourless and purple in colour.
It’s aqueous solution, a sweet taste.
It is soluble in water, pyridine, methanol and other organic solvents.
It has a strong oxidizing property so it is used as an oxidant in many reactions.
Complete Step By Step Answer:
The given molecular formula is- $ C{H_3} - C\left( {C{H_3}} \right) = CH - C{H_3} $
It is also called is isopentane and its IUPAC name is $ 3 - methylbut - 2 - ene $
This compound reacts with given reagents- sodium per-iodate and potassium permanganate. . The aqueous solution of both the reagent is known as Lemieux reagent
We have to find the product.
Here, $ \;KMn{O_4} $ is oxidizing agent so it oxidizes the alkene and cis-diol product is obtained. The reaction is given as-
$ C{H_3} - C\left( {C{H_3}} \right) = CH - C{H_3}\xrightarrow{{KMn{O_4}}}C{H_3} - C\left( {C{H_3}} \right)\left( {OH} \right) - CH\left( {OH} \right) - C{H_3} $
This obtained product is then cleaved by sodium periodate into aldehyde and ketone. The reaction is given as-
$ C{H_3} - C\left( {C{H_3}} \right)\left( {OH} \right) - CH\left( {OH} \right) - C{H_3}\xrightarrow[{ - {H_2}O}]{{NaI{O_4}}}C{H_3}COC{H_3} + C{H_3}CHO $
Now, since aldehyde is more reactive than ketone it is further oxidized by $ KMn{O_4} $ to carboxylic acid. The reaction is given as-
$ C{H_3}CHO\xrightarrow{{KMn{O_4}}}C{H_3}COOH $
The whole reaction can be summarized as-

Hence correct answer is option B.
Note :
Students may go wrong if they think that the reaction stops when aldehyde is formed and choose the option C which is incorrect.
We know that $ KMn{O_4} $ is a strong oxidizing agent which can oxidize the reagent in any medium whether it is acidic, basic or neutral so it further oxidizes aldehyde to carboxylic acid. $ KMn{O_4} $ Has following properties-
It is odourless and purple in colour.
It’s aqueous solution, a sweet taste.
It is soluble in water, pyridine, methanol and other organic solvents.
It has a strong oxidizing property so it is used as an oxidant in many reactions.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




