
The compound,
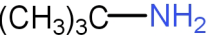 is a :
is a :
(A) Primary amine
(B) Secondary amine
(C) Tertiary amine
(D) Quaternary ammonium salt
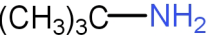
Answer
599.7k+ views
Hint: Try to recall that amines are organic molecules containing nitrogen and hydrogen. When one or more of the hydrogen’s of ammonia are substituted by organic groups they are called amines. Now, by using this you can easily find the correct option.
Complete answer:
It is known to you that depending on how many hydrogen atoms have been replaced by the alkyl group, amines are classified as primary, secondary and tertiary amines. They are as follows:
* Primary amines: Primary amines are formed when one of three hydrogen atoms in ammonia is substituted by an alkyl group which means the formula of the primary amine will be \[RN{H_2}\] where “R” is an alkyl group.
* Secondary amines: In secondary amine, two of hydrogen atoms in an ammonia molecule have been replaced by alkyl groups. The formula of secondary amine will be \[{R_2}NH\].
* Tertiary amines: In a tertiary amine, all of the hydrogen atoms in an ammonia molecule have been replaced by alkyl groups. The formula of tertiary amine will be \[{R_3}N\].
* Quaternary ammonium salt: In a quaternary ammonium salt, nitrogen atom is joined to four alkyl groups. The formula of quaternary ammonium salt will be \[{R_4}{N^ + }\].
* Now, coming to the question the given compound is of type \[RN{H_2}\]. So, it is primary amine.
Hence, from above we can easily conclude that option A is the correct option to the given question.
Note:
* It should be remembered to you that aliphatic amines are more basic than aromatic amines and the basicity of both aliphatic and aromatic amines is due to the presence of lone pair on nitrogen atom.
* Also, you should remember that amines are produced naturally in living things, often as neurotransmitters such as dopamine and histamine.
Complete answer:
It is known to you that depending on how many hydrogen atoms have been replaced by the alkyl group, amines are classified as primary, secondary and tertiary amines. They are as follows:
* Primary amines: Primary amines are formed when one of three hydrogen atoms in ammonia is substituted by an alkyl group which means the formula of the primary amine will be \[RN{H_2}\] where “R” is an alkyl group.
* Secondary amines: In secondary amine, two of hydrogen atoms in an ammonia molecule have been replaced by alkyl groups. The formula of secondary amine will be \[{R_2}NH\].
* Tertiary amines: In a tertiary amine, all of the hydrogen atoms in an ammonia molecule have been replaced by alkyl groups. The formula of tertiary amine will be \[{R_3}N\].
* Quaternary ammonium salt: In a quaternary ammonium salt, nitrogen atom is joined to four alkyl groups. The formula of quaternary ammonium salt will be \[{R_4}{N^ + }\].
* Now, coming to the question the given compound is of type \[RN{H_2}\]. So, it is primary amine.
Hence, from above we can easily conclude that option A is the correct option to the given question.
Note:
* It should be remembered to you that aliphatic amines are more basic than aromatic amines and the basicity of both aliphatic and aromatic amines is due to the presence of lone pair on nitrogen atom.
* Also, you should remember that amines are produced naturally in living things, often as neurotransmitters such as dopamine and histamine.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




