
The compound ${CH_{3}}{CH_{3}}C=CH{CH_{3}}$ on reaction with $NaI{O_{4}}$ in presence of $KMn{O_{4}}$ gives:
a.) ${CH_{3}}CHO+C{O_{2}}$
b.) ${CH_{3}}CO{CH_{3}}$
c.) ${CH_{3}}CO{CH_{3}}+{CH_{3}}COOH$
d.) ${CH_{3}}CO{CH_{3}}+{CH_{3}}CHO$
Answer
548.1k+ views
Hint: When an aqueous solution of sodium periodate and some trace amount of potassium permanganate are combined, they together form a Lemieux reagent. This reagent allows oxidation of alkenes which gives aldehydes or ketones as products.
Complete step by step answer:
- The Lemieux reagent performs oxidation of alkenes to give aldehyde or ketone as products.
- This process is equivalent to that of reductive ozonolysis.
- The given to us reacts with sodium periodate in the presence of potassium permanganate. - In the case of strong oxidizing conditions, like using $KMn{O_{4}}$ instead of osmium tetroxide.
- Potassium permanganate is a strong oxidizing agent. It has the ability to react with various functional groups which include alkenes, sulphides, alcohols, aldehydes, etc.
- $KMn{O_{4}}$ is known to cause cis hydroxylation easily. Apart from this, it oxidizes any aldehydes formed into carboxylic groups.
- Sodium periodate breaks apart the vicinal diols in order to form aldehydes and ketones.
- The reaction in consideration is shown below:
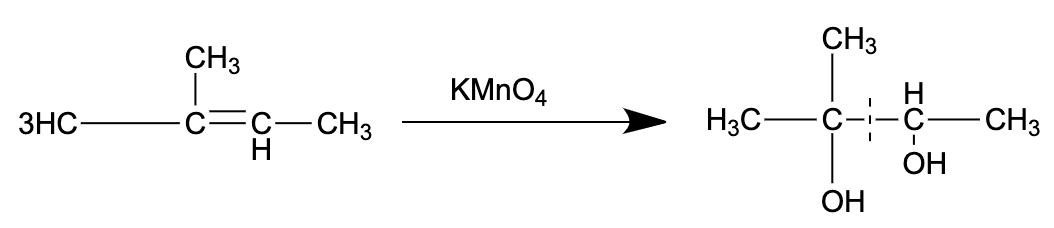
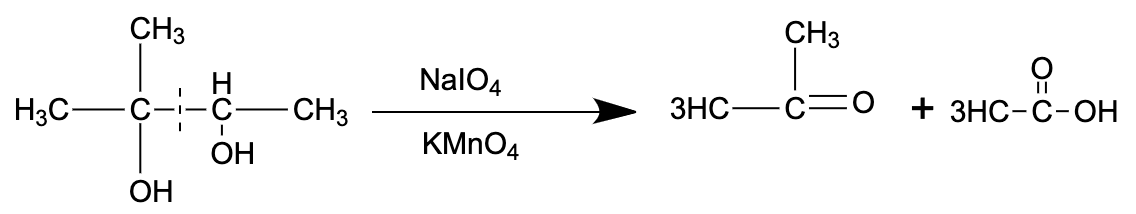
So, the correct answer is “Option C”.
Additional Information”. The conversion of an olefin into two individual aldehydes by the means of oxidative cleavage of a carbon‐carbon double bond with osmium tetroxide and sodium periodate is known as the Lemieux–Johnson oxidative cleavage and the combination of osmium tetroxide and sodium periodate is referred to as Lemieux–Johnson reagent.
Note: The conversion of an olefin into two individual aldehydes by the means of oxidative cleavage of a carbon‐carbon double bond with osmium tetroxide and sodium periodate is known as the Lemieux–Johnson oxidative cleavage and the combination of osmium tetroxide and sodium periodate is referred to as Lemieux–Johnson reagent.
Complete step by step answer:
- The Lemieux reagent performs oxidation of alkenes to give aldehyde or ketone as products.
- This process is equivalent to that of reductive ozonolysis.
- The given to us reacts with sodium periodate in the presence of potassium permanganate. - In the case of strong oxidizing conditions, like using $KMn{O_{4}}$ instead of osmium tetroxide.
- Potassium permanganate is a strong oxidizing agent. It has the ability to react with various functional groups which include alkenes, sulphides, alcohols, aldehydes, etc.
- $KMn{O_{4}}$ is known to cause cis hydroxylation easily. Apart from this, it oxidizes any aldehydes formed into carboxylic groups.
- Sodium periodate breaks apart the vicinal diols in order to form aldehydes and ketones.
- The reaction in consideration is shown below:


So, the correct answer is “Option C”.
Additional Information”. The conversion of an olefin into two individual aldehydes by the means of oxidative cleavage of a carbon‐carbon double bond with osmium tetroxide and sodium periodate is known as the Lemieux–Johnson oxidative cleavage and the combination of osmium tetroxide and sodium periodate is referred to as Lemieux–Johnson reagent.
Note: The conversion of an olefin into two individual aldehydes by the means of oxidative cleavage of a carbon‐carbon double bond with osmium tetroxide and sodium periodate is known as the Lemieux–Johnson oxidative cleavage and the combination of osmium tetroxide and sodium periodate is referred to as Lemieux–Johnson reagent.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




