
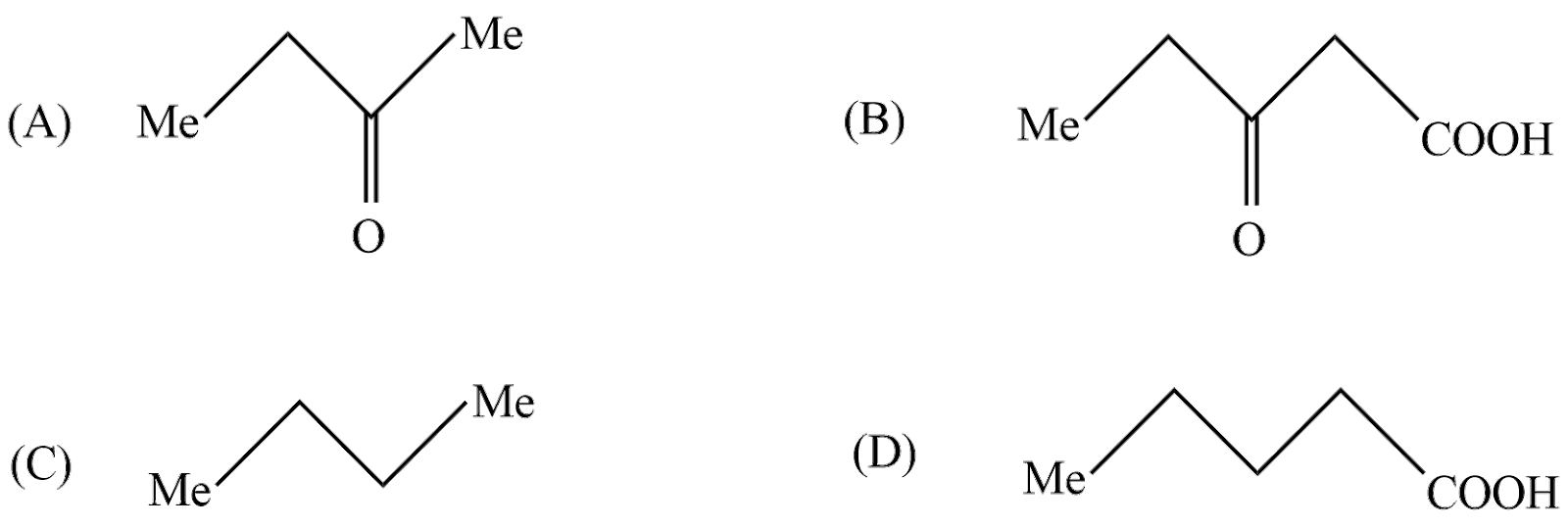
The compound (C) is:
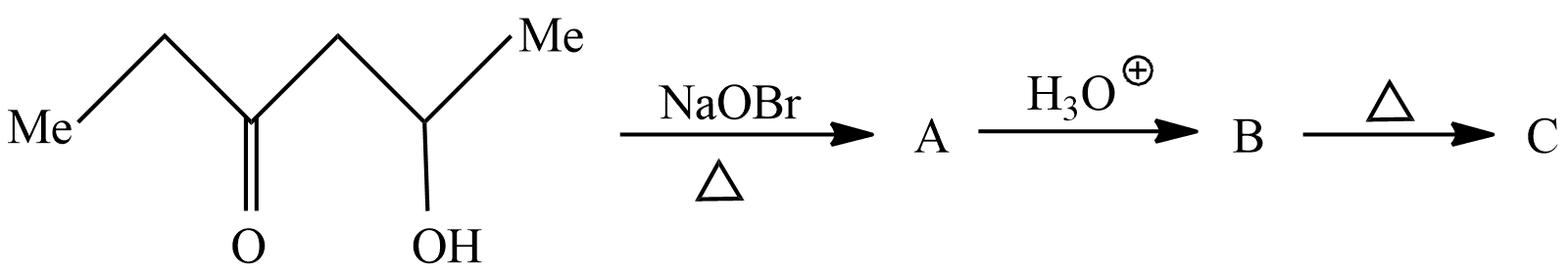
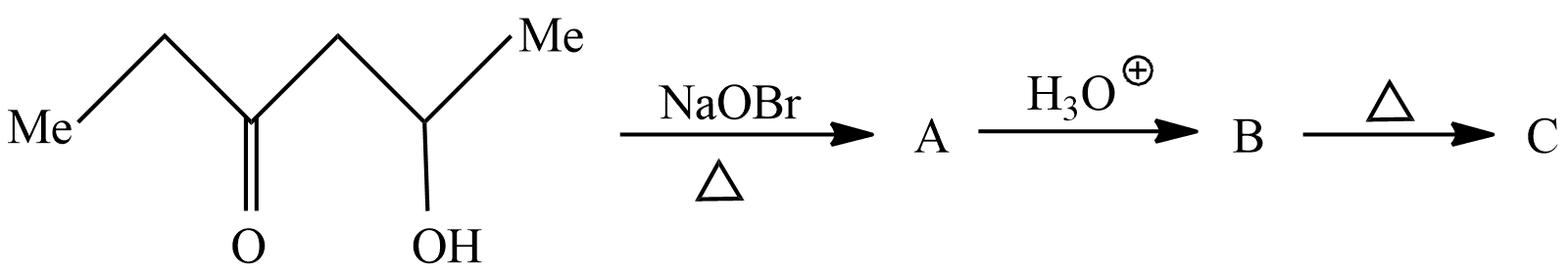

Answer
509.7k+ views
Hint :The compound given here is $ 5 - Hydroxy - 3 - hexanone $ which is treated with Sodium hypobromite to give product A which then is treated with hydronium ion to give product B. Product B is heated to obtain product C. we will see each reaction to understand the process and identify the products formed from each of the reaction.
Complete Step By Step Answer:
In the first reaction $ 5 - Hydroxy - 3 - hexanone is treated with Sodium hypobromite it will remove a methyl group and form alkyl halide and Sodium Hydroxide. The reaction taking place here is written as
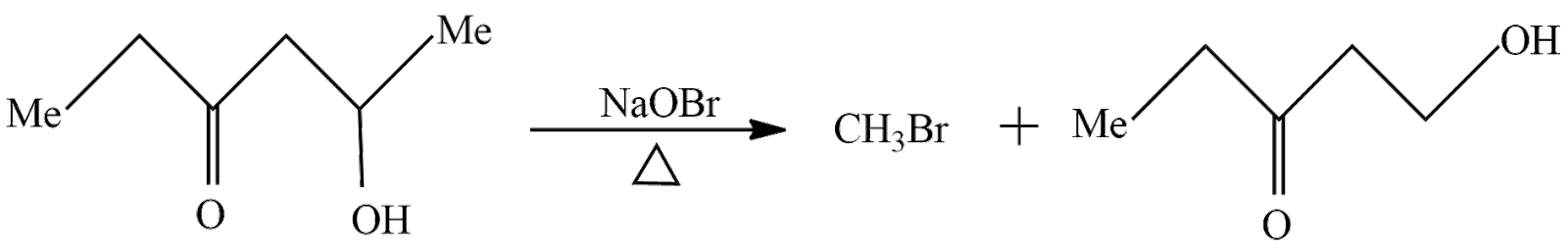
In the above reaction $ 5 - Hydroxy - 3 - hexanone $ reacts with Sodium hypobromite to give Methyl bromide and $ 5 - Hydroxy - 3 - pentanone $ . So, the product A according to reaction is $ 5 - Hydroxy - 3 - pentanone $ .
Let's see the second reaction in which product A reacts with hydronium ion; in an oxidation reaction the alcohol is converted to carboxylic acid. The reaction taking place here is written as
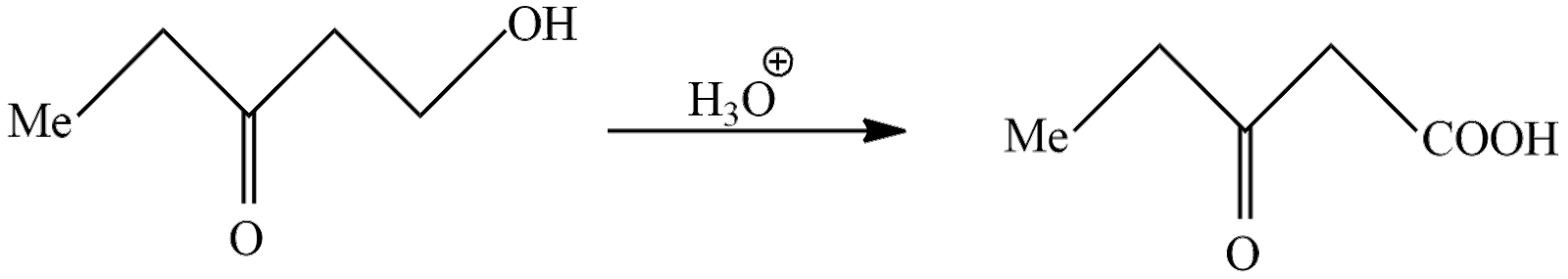
In above reaction 5-hydroxy-2-pentanone undergoes oxidation reaction to form $ 3 - Oxopentanoicacid $ . So, the product B according to question is $ 5 - Hydroxy - 3 - pentanone $
Let's see the last reaction in which product B undergoes strong heating to remove carbon dioxide. It is also known as the decarboxylation reaction. the reaction taking place here is written as
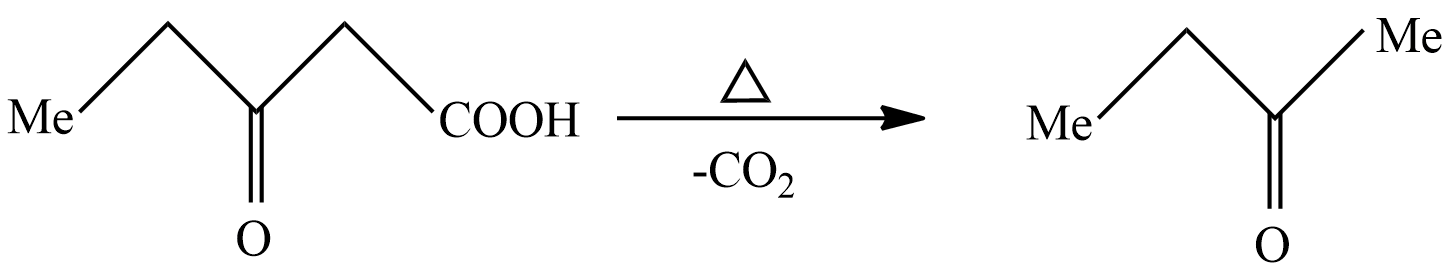
In above reaction $ 3 - $ Oxopentanoicacid undergoes decarboxylation reaction in strong heating conditions to form Carbon dioxide and $ {\text{n}} - butanone $ . So, according to the question the product C is $ {\text{n}} - butanone $ .
Note :
Carefully observe the position of the functional group while writing a reaction because it is necessary for the product formation. Also pay attention to the conditions in which the reaction is carried out because it can affect the reaction.
Complete Step By Step Answer:
In the first reaction $ 5 - Hydroxy - 3 - hexanone is treated with Sodium hypobromite it will remove a methyl group and form alkyl halide and Sodium Hydroxide. The reaction taking place here is written as
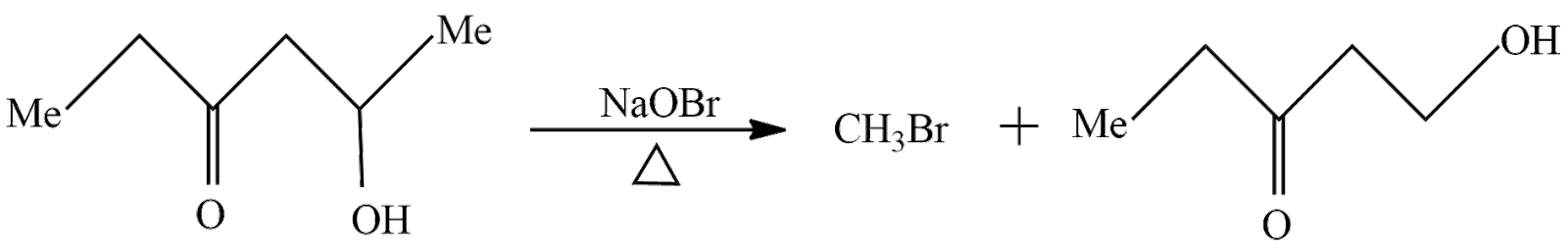
In the above reaction $ 5 - Hydroxy - 3 - hexanone $ reacts with Sodium hypobromite to give Methyl bromide and $ 5 - Hydroxy - 3 - pentanone $ . So, the product A according to reaction is $ 5 - Hydroxy - 3 - pentanone $ .
Let's see the second reaction in which product A reacts with hydronium ion; in an oxidation reaction the alcohol is converted to carboxylic acid. The reaction taking place here is written as

In above reaction 5-hydroxy-2-pentanone undergoes oxidation reaction to form $ 3 - Oxopentanoicacid $ . So, the product B according to question is $ 5 - Hydroxy - 3 - pentanone $
Let's see the last reaction in which product B undergoes strong heating to remove carbon dioxide. It is also known as the decarboxylation reaction. the reaction taking place here is written as
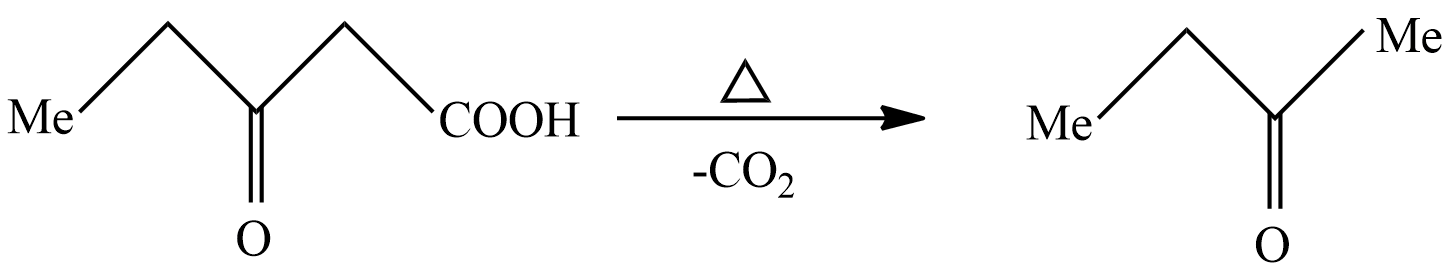
In above reaction $ 3 - $ Oxopentanoicacid undergoes decarboxylation reaction in strong heating conditions to form Carbon dioxide and $ {\text{n}} - butanone $ . So, according to the question the product C is $ {\text{n}} - butanone $ .
Note :
Carefully observe the position of the functional group while writing a reaction because it is necessary for the product formation. Also pay attention to the conditions in which the reaction is carried out because it can affect the reaction.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




