
The compound $\text{B}{{\text{F}} _ {3}} $ is:
This question has multiple correct options
A. Electron deficient compound
B. Lewis acid
C. Used as rocket fuel
D. Ionic compound
Answer
569.4k+ views
Hint: $\text{B}{{\text{F}} _ {3}} $is compound having incomplete octet and due to the presence of empty p-orbitals, it has the tendency to gain the electrons from other donor atoms. Now answer the above statement.
Complete step by step answer:
In $\text{B}{{\text{F}} _ {3}}$, boron has an atomic number as five and a total number of three electrons in its outermost shell. On the other hand, F is halogen and has the atomic number as nine and has a total of seven electrons in its outermost shell. During the formation of boron trifluoride, due to the presence of nine electrons in fluorine, the boron can easily attack the fluorine to complete its octet i.e. the total no of eight electrons in its valence shell. So, the 2s atomic orbital of boron and one 2p atomic orbital of boron i.e. the three electrons of boron forms three bonds with fluorine atoms and spilt the three valence electrons equally among themselves to complete the octet of boron. But there are only three electrons with boron and so only forms three bonds and thus, have a total number of six electrons only i.e. they have incomplete octet. Thus, $\text{B}{{\text{F}} _ {3}} $ is electron deficient in nature.
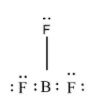
Further, the bond in $\text{B}{{\text{F}} _ {3}} $, is covalent in nature as and is formed by the sharing of electrons and not by the loss or gain of electrons and is thus, a covalent compound.
The $\text{B}{{\text{F}}_{3}}$as we know have three electrons, two electrons in2s atomic orbital and 1 electron in 2p atomic orbitals and the two 2p atomic orbitals are empty and can accept a pair of electrons and thus, therefore act as Lewis acid and results in the formation of coordinate bond.
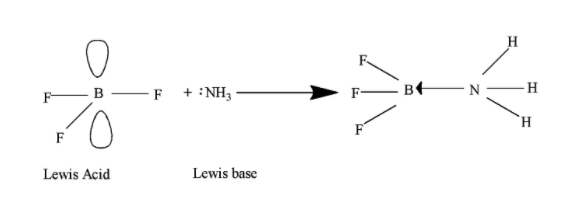
The $\text{B}{{\text{F}} _ {3}} $covalent compound is not used as rocket fuel but the element boron is used as fuel in rockets due to low molecular weight i.e,10.81 and its ability to produce high energy of combustion.
Hence, the options (A) and (B) are correct.
Note: Don’t get confused in the words Lewis acid and Lewis base. Lewis are those which can accept a pair of electrons. e. g. $\text{B}{{\text{F}} _ {3}} $, ${{\text{H}} ^ {+}} $ etc. And on the other hand, Lewis bases are those which can donate a pair of electrons. $\text{N}{{\text{H}}_{3}}$, $\text{O}{{\text{H}}^{-}}$ etc. the Lewis strength of $\text{B}{{\text{F}}_{3}}$ decreases due to the 2p$\pi $-2p$\pi $ back bonding between the 2p orbitals of boron and fluorine atoms.
Complete step by step answer:
In $\text{B}{{\text{F}} _ {3}}$, boron has an atomic number as five and a total number of three electrons in its outermost shell. On the other hand, F is halogen and has the atomic number as nine and has a total of seven electrons in its outermost shell. During the formation of boron trifluoride, due to the presence of nine electrons in fluorine, the boron can easily attack the fluorine to complete its octet i.e. the total no of eight electrons in its valence shell. So, the 2s atomic orbital of boron and one 2p atomic orbital of boron i.e. the three electrons of boron forms three bonds with fluorine atoms and spilt the three valence electrons equally among themselves to complete the octet of boron. But there are only three electrons with boron and so only forms three bonds and thus, have a total number of six electrons only i.e. they have incomplete octet. Thus, $\text{B}{{\text{F}} _ {3}} $ is electron deficient in nature.
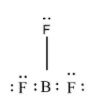
Further, the bond in $\text{B}{{\text{F}} _ {3}} $, is covalent in nature as and is formed by the sharing of electrons and not by the loss or gain of electrons and is thus, a covalent compound.
The $\text{B}{{\text{F}}_{3}}$as we know have three electrons, two electrons in2s atomic orbital and 1 electron in 2p atomic orbitals and the two 2p atomic orbitals are empty and can accept a pair of electrons and thus, therefore act as Lewis acid and results in the formation of coordinate bond.

The $\text{B}{{\text{F}} _ {3}} $covalent compound is not used as rocket fuel but the element boron is used as fuel in rockets due to low molecular weight i.e,10.81 and its ability to produce high energy of combustion.
Hence, the options (A) and (B) are correct.
Note: Don’t get confused in the words Lewis acid and Lewis base. Lewis are those which can accept a pair of electrons. e. g. $\text{B}{{\text{F}} _ {3}} $, ${{\text{H}} ^ {+}} $ etc. And on the other hand, Lewis bases are those which can donate a pair of electrons. $\text{N}{{\text{H}}_{3}}$, $\text{O}{{\text{H}}^{-}}$ etc. the Lewis strength of $\text{B}{{\text{F}}_{3}}$ decreases due to the 2p$\pi $-2p$\pi $ back bonding between the 2p orbitals of boron and fluorine atoms.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




