
The compound B formed in the following sequences of reactions is
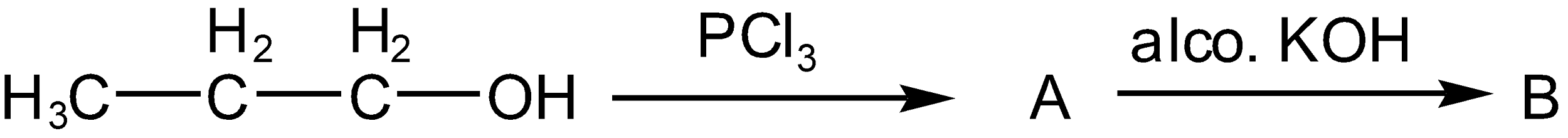
A. Propyne
B. Propene
C. Propanol
D. Propane
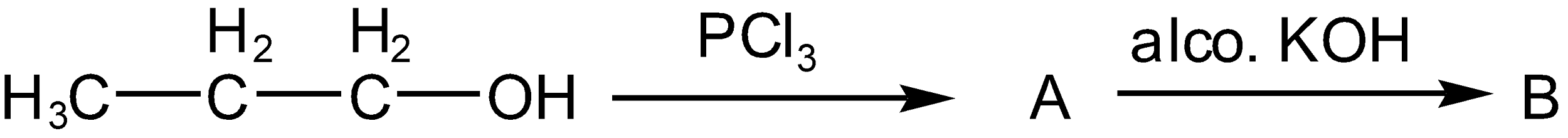
Answer
362.7k+ views
Hint: In the given question, alcohol (OH group represents alcohol) is made to react with \[PC{{l}_{3}}\], phosphorus trichloride. The reagent \[PC{{l}_{3}}\] is quite toxic and very reactive and reacts with alcohol to give alkyl halide and phosphorus acid. In the next step, alkyl halide is made to react with alcoholic KOH. The alcohol KOH acts as a strong base thus, when it reacts with alkyl halide it eliminates halide from alpha hydrogen and proton from beta carbon to generate alkene.
Complete Step by Step Answer:
Firstly the alcohol which is given is propanol (ROH where R is propane, chain of three carbon) is made to react with \[PC{{l}_{3}}\] which is very reactive and thus after reaction, it will change alcoholic group of propane to chloride group such as propyl chloride (RCl where R is propane) and acid of phosphoric acid (\[{{H}_{3}}P{{O}_{3}}\]) such as
The general equation is given as
As per question,
The resulting propyl chloride (alkyl halide) reacts with alco. KOH. As KOH behaves as a base thus, it releases \[O{{H}^{-}}\]and \[{{K}^{+}}\]ions in the presence of propyl chloride. Now chloride ion which is negatively charged in propyl chloride form bond with positively charged potassium, \[{{K}^{+}}\] and carbocation (on alpha carbon) will generate such as
Now the negatively charged OH which is a strong base (tendency to donate an electron to electron-deficient species mostly to the proton) attacks on proton on beta carbon so that an extra electron on beta carbon compensatesfor the loss of one electron on alpha carbon and this result in the formationof a pi bond. Thus, double bond will form between alpha and beta carbon and can say the formation of propene (alkene) will take place such as
Thus, the correct option is B.
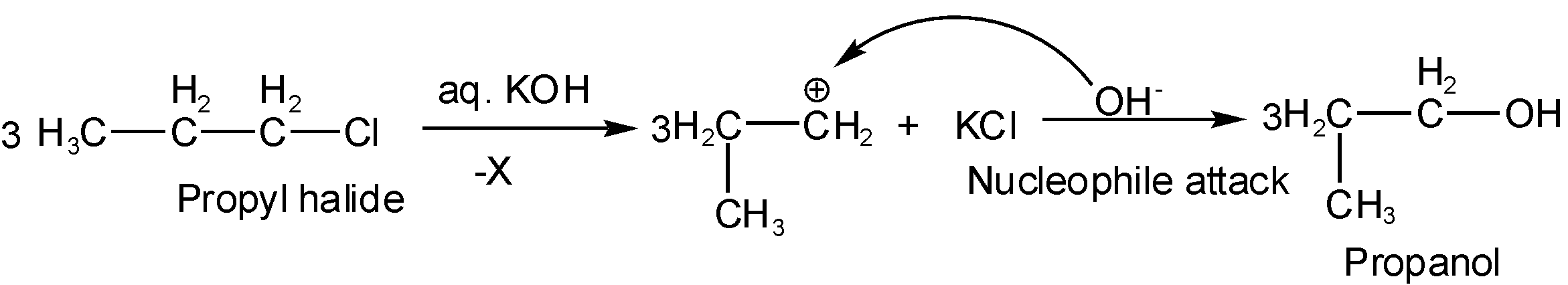
Note: It is important to note that if in the place of alcohol. KOH, aq KOH is present that negative ion of KOH, \[O{{H}^{-}}\] behave like a nucleophile and thus attack carbonation generated in middle directly and again the formation of alcohol will take place and reaction is said to be nucleophile substitution reaction such as
Alpha carbon is one at which substituent or any functional group is present and next to it is the beta carbon and next to beta carbon, carbon is said to gamma carbon.
Complete Step by Step Answer:
Firstly the alcohol which is given is propanol (ROH where R is propane, chain of three carbon) is made to react with \[PC{{l}_{3}}\] which is very reactive and thus after reaction, it will change alcoholic group of propane to chloride group such as propyl chloride (RCl where R is propane) and acid of phosphoric acid (\[{{H}_{3}}P{{O}_{3}}\]) such as
The general equation is given as
As per question,
The resulting propyl chloride (alkyl halide) reacts with alco. KOH. As KOH behaves as a base thus, it releases \[O{{H}^{-}}\]and \[{{K}^{+}}\]ions in the presence of propyl chloride. Now chloride ion which is negatively charged in propyl chloride form bond with positively charged potassium, \[{{K}^{+}}\] and carbocation (on alpha carbon) will generate such as
Now the negatively charged OH which is a strong base (tendency to donate an electron to electron-deficient species mostly to the proton) attacks on proton on beta carbon so that an extra electron on beta carbon compensatesfor the loss of one electron on alpha carbon and this result in the formationof a pi bond. Thus, double bond will form between alpha and beta carbon and can say the formation of propene (alkene) will take place such as
Thus, the correct option is B.
Note: It is important to note that if in the place of alcohol. KOH, aq KOH is present that negative ion of KOH, \[O{{H}^{-}}\] behave like a nucleophile and thus attack carbonation generated in middle directly and again the formation of alcohol will take place and reaction is said to be nucleophile substitution reaction such as

Alpha carbon is one at which substituent or any functional group is present and next to it is the beta carbon and next to beta carbon, carbon is said to gamma carbon.
Recently Updated Pages
Master Class 8 Social Science: Engaging Questions & Answers for Success

Master Class 8 English: Engaging Questions & Answers for Success

Class 8 Question and Answer - Your Ultimate Solutions Guide

Master Class 8 Maths: Engaging Questions & Answers for Success

Master Class 8 Science: Engaging Questions & Answers for Success

Master Class 7 English: Engaging Questions & Answers for Success

Trending doubts
What are the factors of 100 class 7 maths CBSE

Which are the Top 10 Largest Countries of the World?

What is BLO What is the full form of BLO class 8 social science CBSE

The value of 6 more than 7 is A 1 B 1 C 13 D 13 class 7 maths CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE




