
The components A and B in the following reaction are, respectively :
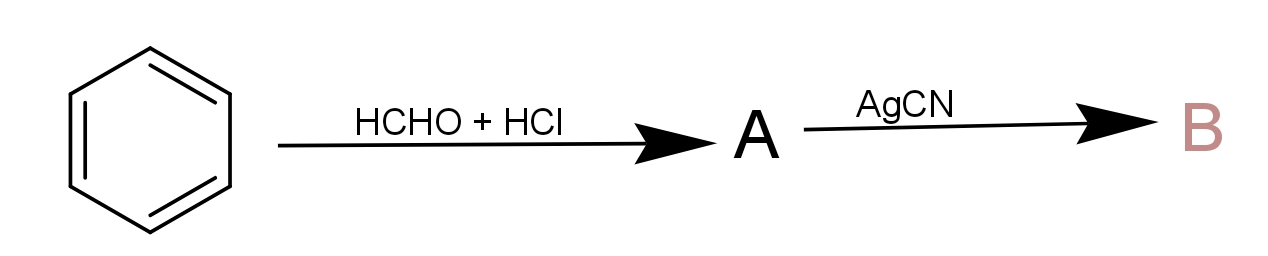
a.) A = Benzyl alcohol, B = Benzyl isocyanide
b.) A = Benzyl alcohol, B = Benzyl cyanide
c.) A = Benzyl chloride, B = Benzyl cyanide
d.) A = Benzyl chloride, B = Benzyl isocyanide

Answer
565.5k+ views
Hint: The benzene is electron rich and undergoes electrophilic substitution reactions. The product A formed here is similar to that produced in Blanc chloromethylation reaction. The AgCN reacts with A to give B along with AgCl.
Complete step by step answer:
In the reaction, we have been given benzene as the substrate and the reagents are formaldehyde and hydrochloric acid. This reaction is chloromethylation of benzene.
The benzene reacts with formaldehyde in the presence of HCl to give benzene methyl chloride. It is an electrophilic substitution reaction.
-Let us see the reaction step by step.
Step 1 : The HCHO reacts with HCl to give $ \oplus C{H_2}Cl$. This species is electrophile.
Step 2 : In the second step the electrophile produced in step 1 i.e. $ \oplus C{H_2}Cl$ will react with benzene to given benzyl chloride. This product is A.
After this, the product A reacts with AgCN to give benzyl isocyanide as-
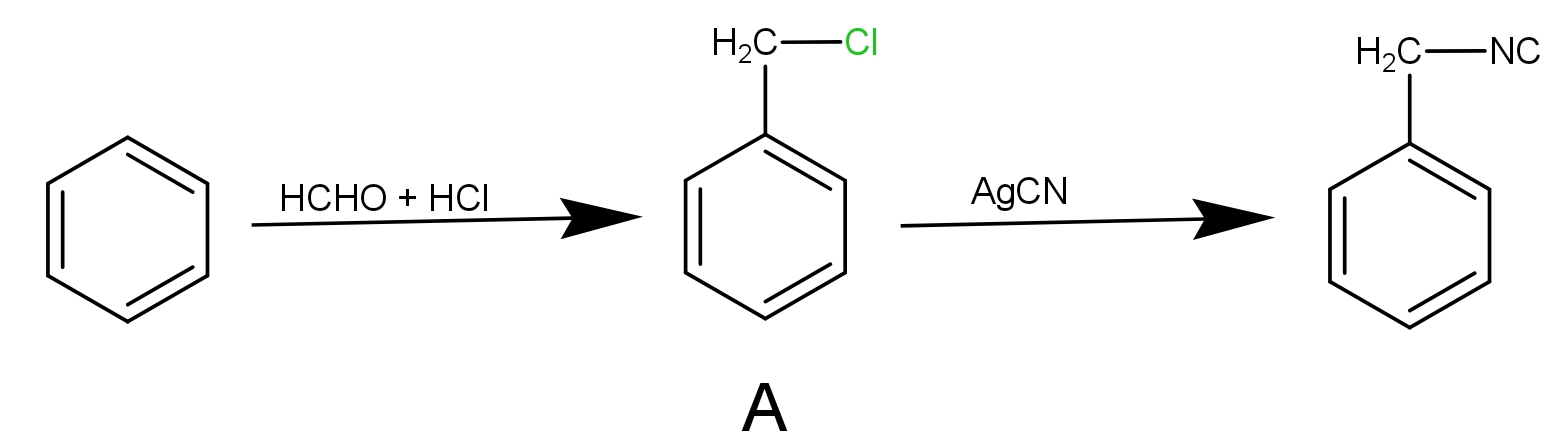
The AgCN is covalent in nature. The ‘C’ and ‘N’ both can donate the electrons but the attack takes place mainly through ‘N’ as it can donate the electron pair giving rise to isocyanide product as the major. So, the product B is benzyl isocyanide.
Thus, the option d.) is the correct answer.
Note: It must be noted that the best method to perform chloromethylation of benzene is the Blanc reaction. In this method, along with HCHO and hydrochloric acid, a lewis acid like $ZnC{l_2}$ is used. The acidic conditions are to protonate the HCHO carbonyl. This will make it more electrophilic and thus the reaction proceeds easily. If instead of AgCN, there is KCN, then the product would have been cyanide because KCN is ionic in nature.
Complete step by step answer:
In the reaction, we have been given benzene as the substrate and the reagents are formaldehyde and hydrochloric acid. This reaction is chloromethylation of benzene.
The benzene reacts with formaldehyde in the presence of HCl to give benzene methyl chloride. It is an electrophilic substitution reaction.
-Let us see the reaction step by step.
Step 1 : The HCHO reacts with HCl to give $ \oplus C{H_2}Cl$. This species is electrophile.
Step 2 : In the second step the electrophile produced in step 1 i.e. $ \oplus C{H_2}Cl$ will react with benzene to given benzyl chloride. This product is A.
After this, the product A reacts with AgCN to give benzyl isocyanide as-

The AgCN is covalent in nature. The ‘C’ and ‘N’ both can donate the electrons but the attack takes place mainly through ‘N’ as it can donate the electron pair giving rise to isocyanide product as the major. So, the product B is benzyl isocyanide.
Thus, the option d.) is the correct answer.
Note: It must be noted that the best method to perform chloromethylation of benzene is the Blanc reaction. In this method, along with HCHO and hydrochloric acid, a lewis acid like $ZnC{l_2}$ is used. The acidic conditions are to protonate the HCHO carbonyl. This will make it more electrophilic and thus the reaction proceeds easily. If instead of AgCN, there is KCN, then the product would have been cyanide because KCN is ionic in nature.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




