
The colour of sodium chromate is:
A.Black
B.Red
C.Yellow
D.Green
Answer
577.2k+ views
Hint: Sodium chromate is an inorganic compound. The molecular formula for sodium chromate is ${\text{N}}{{\text{a}}_{\text{2}}}{\text{Cr}}{{\text{O}}_{\text{4}}}$. Sodium chromate is also known as chromic acid. The colour of sodium chromate is the same as that of potassium chromate.
Complete step by step answer:
-Sodium chromate is an inorganic compound having molecular formula ${\text{N}}{{\text{a}}_{\text{2}}}{\text{Cr}}{{\text{O}}_{\text{4}}}$. Sodium chromate is an intermediate in extracting chromium from its ores.
-The structure of sodium chromate is as follows:
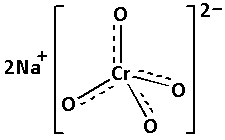
-Sodium chromate can be obtained by roasting of chromium ores in the air in the presence of sodium carbonate.
-Sodium chromate is odourless and it is slightly soluble in ethanol.
-Sodium chromate appears as yellow crystals. Thus, the colour of sodium chromate is yellow.
So, the correct answer is “Option C”.
Additional Information:
The uses of sodium chromate are as follows:
1.Sodium chromate is used in extraction of chromium from its ores.
2.Sodium chromate is used in the petroleum industry to inhibit corrosion.
3.Sodium chromate is used in the textile industry for dyeing because of its bright yellow colour.
4.Sodium chromate is used in pharmaceuticals to determine the volume of red blood cells.
5.Sodium chromate is used in organic chemistry as an oxidising agent to convert primary alcohols to carboxylic acids and secondary alcohols to ketones.
Note:
Sodium chromate is a carcinogenic substance i.e. it can cause cancer. Also, sodium chromate is corrosive in nature. Exposure to sodium chromate may cause severe damage to the eyes, blindness, impaired fertility, heritable genetic damage, etc.
Complete step by step answer:
-Sodium chromate is an inorganic compound having molecular formula ${\text{N}}{{\text{a}}_{\text{2}}}{\text{Cr}}{{\text{O}}_{\text{4}}}$. Sodium chromate is an intermediate in extracting chromium from its ores.
-The structure of sodium chromate is as follows:

-Sodium chromate can be obtained by roasting of chromium ores in the air in the presence of sodium carbonate.
-Sodium chromate is odourless and it is slightly soluble in ethanol.
-Sodium chromate appears as yellow crystals. Thus, the colour of sodium chromate is yellow.
So, the correct answer is “Option C”.
Additional Information:
The uses of sodium chromate are as follows:
1.Sodium chromate is used in extraction of chromium from its ores.
2.Sodium chromate is used in the petroleum industry to inhibit corrosion.
3.Sodium chromate is used in the textile industry for dyeing because of its bright yellow colour.
4.Sodium chromate is used in pharmaceuticals to determine the volume of red blood cells.
5.Sodium chromate is used in organic chemistry as an oxidising agent to convert primary alcohols to carboxylic acids and secondary alcohols to ketones.
Note:
Sodium chromate is a carcinogenic substance i.e. it can cause cancer. Also, sodium chromate is corrosive in nature. Exposure to sodium chromate may cause severe damage to the eyes, blindness, impaired fertility, heritable genetic damage, etc.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




