
The color of ppt A, B, and C respectively is?
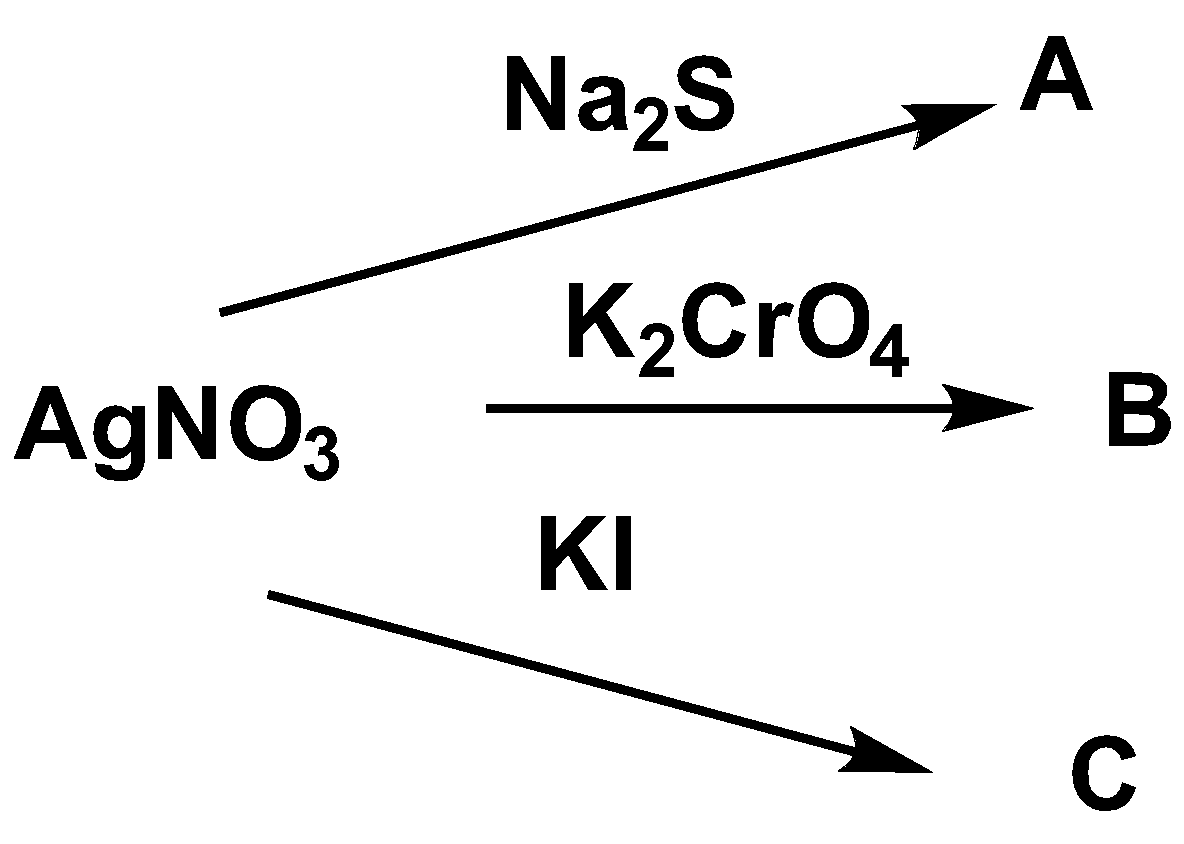
A. Black, yellow, deep yellow
B. Black. red, white
C. Brown, red, white
D. Black, white, red

Answer
585.9k+ views
Hint: Precipitate is basically the creation of a solid form of solution. The chemical which forms the solid is called the precipitate. If an ion is insoluble based on solubility, then it forms a solid(precipitate) with an ion from the other reactant present. While if all the ions in a reaction are soluble, i.e. they will remain in aqueous ion form, then no precipitation will occur.
Complete step by step answer:
When silver nitrate and sodium sulfide react with each other, the black color precipitation of \[A{g_2}S\] is formed. The reaction is shown below.
\[AgN{O_3} + N{a_2}S \to A{g_2}S + NaN{O_3}\]
When silver nitrate and potassium chromate reacts with each other, yellow color precipitation of\[A{g_2}Cr{O_4}\] is formed. The reaction is shown below.
\[2AgN{O_3} + {K_2}Cr{O_4} \to 2KN{O_3} + A{g_2}Cr{O_4}\]
When silver nitrate and potassium iodide reacts with each other, deep yellow color precipitation of AgI is formed. The reaction is shown below.
\[AgN{O_3} + KI \to KN{O_3} + AgI\]
According to reaction, the reactions are,
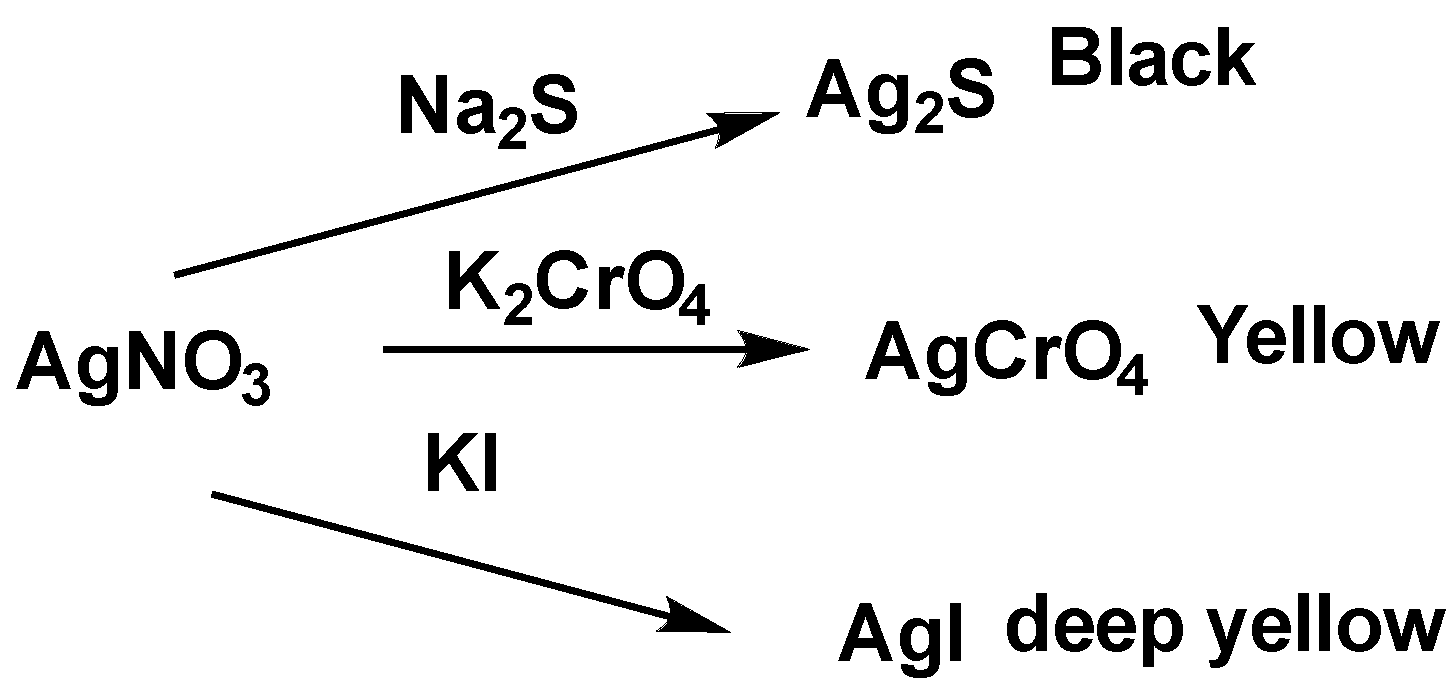
So, the correct option is A.
Additional Information:
An aqueous solution of magnesium chloride and silver nitrate when mixed results in the formation of solid silver chloride and aqueous magnesium nitrate.
We can express this reaction in terms of the molecular equation. It is a balanced chemical equation having molecules rather than ions with subscripts indicating the nature of the compound.
This gives us a balanced molecular equation-
\[MgC{l_2}(aq) + 2AgN{O_3}(aq) \to 2AgCl(s) \downarrow + Mg{(N{O_3})_2}(aq)\]
Note:
The solubility of silver halides is dependent on the ionic size and hydration energy of the halides in the salts. If more energy is released upon hydration than there is stored in the ionic solid lattice, then solubility is favored. If there is a net gain of energy, i.e., if less energy is released on hydration than is stored in the ionic solid lattice, then solubility is unfavored. As we move down the period, Hydration energy decreases. Hence solubility also decreases as we move down a period. Hence, the trend for solubility in halides due to hydration energy can be shown as:
${F^ - } < C{l^ - } < B{r^ - } < {I^ - }$
Hence, the decreasing order of solubility of silver halide is:
\[AgF{\text{ }} > {\text{ }}AgCl{\text{ }} > {\text{ }}AgBr{\text{ }} > {\text{ }}AgI\]
Complete step by step answer:
When silver nitrate and sodium sulfide react with each other, the black color precipitation of \[A{g_2}S\] is formed. The reaction is shown below.
\[AgN{O_3} + N{a_2}S \to A{g_2}S + NaN{O_3}\]
When silver nitrate and potassium chromate reacts with each other, yellow color precipitation of\[A{g_2}Cr{O_4}\] is formed. The reaction is shown below.
\[2AgN{O_3} + {K_2}Cr{O_4} \to 2KN{O_3} + A{g_2}Cr{O_4}\]
When silver nitrate and potassium iodide reacts with each other, deep yellow color precipitation of AgI is formed. The reaction is shown below.
\[AgN{O_3} + KI \to KN{O_3} + AgI\]
According to reaction, the reactions are,

So, the correct option is A.
Additional Information:
An aqueous solution of magnesium chloride and silver nitrate when mixed results in the formation of solid silver chloride and aqueous magnesium nitrate.
We can express this reaction in terms of the molecular equation. It is a balanced chemical equation having molecules rather than ions with subscripts indicating the nature of the compound.
This gives us a balanced molecular equation-
\[MgC{l_2}(aq) + 2AgN{O_3}(aq) \to 2AgCl(s) \downarrow + Mg{(N{O_3})_2}(aq)\]
Note:
The solubility of silver halides is dependent on the ionic size and hydration energy of the halides in the salts. If more energy is released upon hydration than there is stored in the ionic solid lattice, then solubility is favored. If there is a net gain of energy, i.e., if less energy is released on hydration than is stored in the ionic solid lattice, then solubility is unfavored. As we move down the period, Hydration energy decreases. Hence solubility also decreases as we move down a period. Hence, the trend for solubility in halides due to hydration energy can be shown as:
${F^ - } < C{l^ - } < B{r^ - } < {I^ - }$
Hence, the decreasing order of solubility of silver halide is:
\[AgF{\text{ }} > {\text{ }}AgCl{\text{ }} > {\text{ }}AgBr{\text{ }} > {\text{ }}AgI\]
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




