
The closest-packing sequence ABC ABC..... represents
A. primitive cubic packing
B. body-centred cubic packing
C. face-centred cubic packing
D. hexagonal packing
Answer
585.9k+ views
Hint: The closest-packing sequence AAA AAA..represents primitive cubic packing. The closest-packing sequence ABA BAB..in which the third layer is placed into tetrahedral voids represents hexagonal packing and the closest-packing sequence ABC ABC.. with spheres of third layer are placed into octahedral voids, representing the face-centred cubic packing.
Complete step by step answer:
In this question we have been asked about the ABC ABC close packing. So, first we will learn from the ABC ABC type of close packing forms. First we will talk about close packing in one dimension, which can be done by only one way by placing them in one row, touching each other.

Now, we come to close packing in two dimensions. As we are talking about ABC ABC type of close packing so the second row should be different from the first row. So, we place the second row in the depression of the first row which we called hexagonal close packing.
Now, let's take a layer of hexagonal close packing and place a similar layer in such a way that the second layer fits into the depression of the first layer. Let's call the 1st layer A and second layer B.
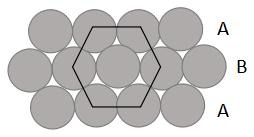
Now we can see that tetrahedral and octahedral voids are formed. So, the third layer can be placed in two ways. One is placing a third layer on tetrahedral voids, by which we get AB AB type of close packing and we call it hexagonal close packing. The second way is placing the third layer of octahedral voids, by this way we get ABC ABC type of packing and we call it face
centred cubic packing.
So, the correct answer will be C. face-centred cubic packing.
Note:
Body centered packing is cubes with atoms at each corner and an atom in the center of every cube. The corner atom is shared between 8 unit cells.Body-centered cubic (BCC) is the name given to a type of atom arrangement found in nature. A body-centered cubic unit cell structure consists of atoms arranged in a cube where each corner of the cube shares an atom and with one atom positioned at the center.
Complete step by step answer:
In this question we have been asked about the ABC ABC close packing. So, first we will learn from the ABC ABC type of close packing forms. First we will talk about close packing in one dimension, which can be done by only one way by placing them in one row, touching each other.

Now, we come to close packing in two dimensions. As we are talking about ABC ABC type of close packing so the second row should be different from the first row. So, we place the second row in the depression of the first row which we called hexagonal close packing.
Now, let's take a layer of hexagonal close packing and place a similar layer in such a way that the second layer fits into the depression of the first layer. Let's call the 1st layer A and second layer B.

Now we can see that tetrahedral and octahedral voids are formed. So, the third layer can be placed in two ways. One is placing a third layer on tetrahedral voids, by which we get AB AB type of close packing and we call it hexagonal close packing. The second way is placing the third layer of octahedral voids, by this way we get ABC ABC type of packing and we call it face
centred cubic packing.
So, the correct answer will be C. face-centred cubic packing.
Note:
Body centered packing is cubes with atoms at each corner and an atom in the center of every cube. The corner atom is shared between 8 unit cells.Body-centered cubic (BCC) is the name given to a type of atom arrangement found in nature. A body-centered cubic unit cell structure consists of atoms arranged in a cube where each corner of the cube shares an atom and with one atom positioned at the center.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




