
The chlorine to oxygen bond distance in $ClO_{4}^{-}$ is 1.44 $\overset{\circ }{\mathop{\text{A}}}\,$. From this, we can conclude that there must be a considerable double bond character in the bonds.
If the given statement is true then enter 1, else enter 0.
Answer
534.6k+ views
Hint: In $ClO_{4}^{-}$, the central atom is chlorine and there are four oxygen atoms joined to it. There are seven electrons in the valence shell of chlorine and it has one negative charge, so there was an addition of one electron in the compound. And one oxygen atom is attached through two electrons of the chlorine atom.
Complete step by step solution: In $ClO_{4}^{-}$, the central atom is chlorine and there are four oxygen atoms joined to it. There are seven electrons in the valence shell of chlorine and it has one negative charge, so there was an addition of one electron in the compound. Generally the length of the single bond between chlorine and oxygen is 1.69 $\overset{\circ }{\mathop{\text{A}}}\,$ and the length of the double between chlorine and oxygen is 1.40 $\overset{\circ }{\mathop{\text{A}}}\,$. In $ClO_{4}^{-}$, one oxygen atom is attached through two electrons of the chlorine atom, so in this the length of all the bonds present in the compound is 1.44 $\overset{\circ }{\mathop{\text{A}}}\,$. The structure is given below:
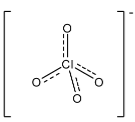
As we can see that there is a partial double bond in all the bonds in the compound, and the bond is near to the bond length of the double bond. Therefore, it is considered a double bond.
Hence, the given statement in the question is true.
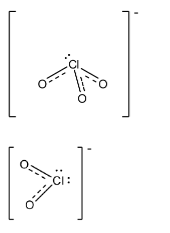
Note: In $ClO_{3}^{-}$ and $ClO_{2}^{-}$, there is also partial double bond character, as in $ClO_{4}^{-}$ and there bond length is also the same as in the $ClO_{4}^{-}$. The structures of both the compounds are given below:
Complete step by step solution: In $ClO_{4}^{-}$, the central atom is chlorine and there are four oxygen atoms joined to it. There are seven electrons in the valence shell of chlorine and it has one negative charge, so there was an addition of one electron in the compound. Generally the length of the single bond between chlorine and oxygen is 1.69 $\overset{\circ }{\mathop{\text{A}}}\,$ and the length of the double between chlorine and oxygen is 1.40 $\overset{\circ }{\mathop{\text{A}}}\,$. In $ClO_{4}^{-}$, one oxygen atom is attached through two electrons of the chlorine atom, so in this the length of all the bonds present in the compound is 1.44 $\overset{\circ }{\mathop{\text{A}}}\,$. The structure is given below:

As we can see that there is a partial double bond in all the bonds in the compound, and the bond is near to the bond length of the double bond. Therefore, it is considered a double bond.
Hence, the given statement in the question is true.
Note: In $ClO_{3}^{-}$ and $ClO_{2}^{-}$, there is also partial double bond character, as in $ClO_{4}^{-}$ and there bond length is also the same as in the $ClO_{4}^{-}$. The structures of both the compounds are given below:

Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




