
The chemical formula of X, Y and Z are:
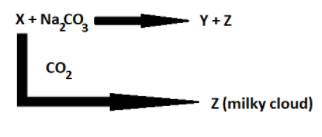
A. $CaO,Ca{\left( {OH} \right)_2},NaOH$
B. $NaOH,CaO,CaC{O_3}$
C. $NaOH,Ca{\left( {OH} \right)_2},CaC{O_3}$
D. $Ca{\left( {OH} \right)_2},NaOH,CaC{O_3}$
Answer
581.7k+ views
Hint: As the options are given in terms of Calcium, Find the compound of calcium which on reacting with Carbon dioxide forms a milky cloud. And then find the milky cloud compound and the products when calcium compound (X) is reacted with Sodium carbonate.
Complete step by step answer:
We are given two equations with unknown compounds and with clues. We have to find the compound names.
The only calcium compound when it is reacted with carbon dioxide turns into a milky cloud is Calcium hydroxide.
Calcium hydroxide is also known as slaked lime or lime water.
Calcium hydroxide reacts with carbon dioxide to form a white precipitate of calcium carbonate and water.
$Ca{\left( {OH} \right)_2} + C{O_2}\xrightarrow[{ - {H_2}O}]{}CaC{O_3}$
The milky cloud is the precipitate of calcium carbonate. Calcium carbonate is also known as Lime stone.
Therefore, X is Calcium hydroxide $Ca{\left( {OH} \right)_2}$ and Z is calcium carbonate $CaC{O_3}$
Now we have to find the compound Y.
X reacts with Sodium carbonate to give Y and Z, this means Calcium hydroxide reacts with Sodium carbonate to form Y and Calcium carbonate.
Writing the equation of reaction of Calcium hydroxide Sodium carbonate
$Ca{\left( {OH} \right)_2} + N{a_2}C{O_3} \to Y + CaC{O_3} \\
\therefore Y = 2NaOH \\
Ca{\left( {OH} \right)_2} + N{a_2}C{O_3} \to 2NaOH + CaC{O_3} \\$
Calcium hydroxide reacts with Sodium carbonate to form 2 moles of Sodium hydroxide and 1 mole of Calcium carbonate.
Therefore, the compound Y is NaOH.
X is $Ca{\left( {OH} \right)_2}$, Y is $NaOH$ and Z is $CaC{O_3}$.
The correct option is Option D, $Ca{\left( {OH} \right)_2},NaOH,CaC{O_3}$.
Note: Do not confuse sodium carbonate $N{a_2}C{O_3}$ with sodium bicarbonate $NaHC{O_3}$ and do not confuse calcium carbonate $CaC{O_3}$ with calcium bicarbonate $CaHC{O_3}$. The names and chemical formulas of these compounds are almost similar. So be careful when dealing with them.
Complete step by step answer:
We are given two equations with unknown compounds and with clues. We have to find the compound names.
The only calcium compound when it is reacted with carbon dioxide turns into a milky cloud is Calcium hydroxide.
Calcium hydroxide is also known as slaked lime or lime water.
Calcium hydroxide reacts with carbon dioxide to form a white precipitate of calcium carbonate and water.
$Ca{\left( {OH} \right)_2} + C{O_2}\xrightarrow[{ - {H_2}O}]{}CaC{O_3}$
The milky cloud is the precipitate of calcium carbonate. Calcium carbonate is also known as Lime stone.
Therefore, X is Calcium hydroxide $Ca{\left( {OH} \right)_2}$ and Z is calcium carbonate $CaC{O_3}$
Now we have to find the compound Y.
X reacts with Sodium carbonate to give Y and Z, this means Calcium hydroxide reacts with Sodium carbonate to form Y and Calcium carbonate.
Writing the equation of reaction of Calcium hydroxide Sodium carbonate
$Ca{\left( {OH} \right)_2} + N{a_2}C{O_3} \to Y + CaC{O_3} \\
\therefore Y = 2NaOH \\
Ca{\left( {OH} \right)_2} + N{a_2}C{O_3} \to 2NaOH + CaC{O_3} \\$
Calcium hydroxide reacts with Sodium carbonate to form 2 moles of Sodium hydroxide and 1 mole of Calcium carbonate.
Therefore, the compound Y is NaOH.
X is $Ca{\left( {OH} \right)_2}$, Y is $NaOH$ and Z is $CaC{O_3}$.
The correct option is Option D, $Ca{\left( {OH} \right)_2},NaOH,CaC{O_3}$.
Note: Do not confuse sodium carbonate $N{a_2}C{O_3}$ with sodium bicarbonate $NaHC{O_3}$ and do not confuse calcium carbonate $CaC{O_3}$ with calcium bicarbonate $CaHC{O_3}$. The names and chemical formulas of these compounds are almost similar. So be careful when dealing with them.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




