
The chemical formula of calcium hypochlorite is:
(A) $COC{{l}_{2}}$
(B) $Ca{{(ClO)}_{2}}$
(C) $Ca{{(Cl{{O}_{3}})}_{2}}$
(D) $Ca{{(Cl{{O}_{4}})}_{2}}$
Answer
582.6k+ views
Hint: in calcium hypochlorite, calcium is bonded to two oxygen atoms and oxygen atom is linked to chlorine atom and calcium. Oxidation number of calcium is +2. Oxygen has oxidation state as -2 and chlorine has oxidation state as +1.
Complete solution step by step:
-In bleaching powder, chlorine powder or chlorinated lime, calcium hypochlorite is the main active ingredient.
-in calcium hypochlorite, calcium is bonded to two oxygen atoms and oxygen atom is linked to chlorine atom and calcium. Oxidation number of calcium is +2. Oxygen has oxidation state as -2 and chlorine has oxidation state as +1.
-structure of calcium hypochlorite is as follows:
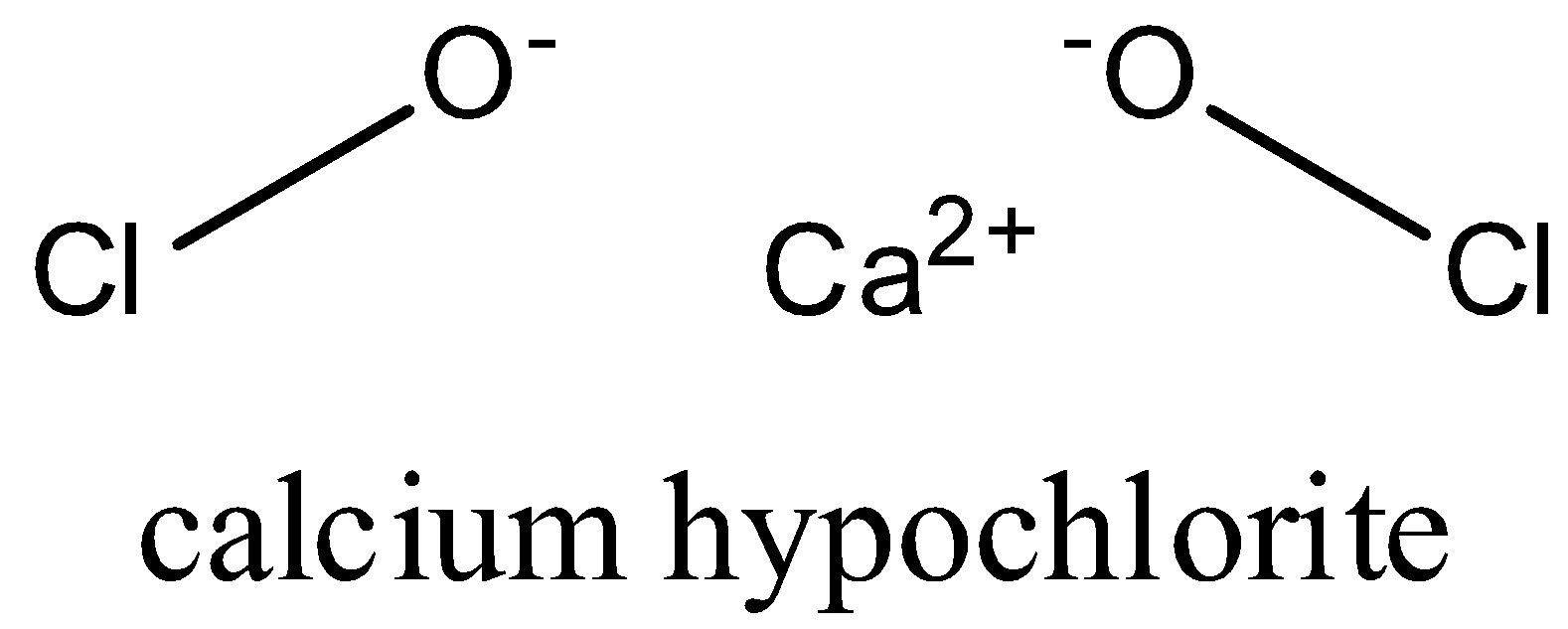
-it is used in water treatment to remove hardness of water and also as a bleaching agent.
-it is an inorganic compound as there is no carbon involved in structure.
- it is more stable and easily available.
-it has a strong smell of chlorine and it decomposes in moist air.
-it has good solubility in soft to medium hard water.
-it is prepared by treating calcium hydroxide known as lime with chlorine gas.
It exists in two forms, dry and hydrated.
-it is commonly used for sanitization of swimming pools. It is a good oxidizing agent.
The chemical formula of calcium hypochlorite is (B) $Ca{{(ClO)}_{2}}$
Note: Calcium hypochlorite is basic in nature. It is used confused with calcium oxychloride. Oxidation number of calcium is +2. Oxygen has oxidation state as -2 and chlorine has oxidation state as +1. It is used as a disinfectant. $COC{{l}_{2}}$ is carbonyl chloride.
Complete solution step by step:
-In bleaching powder, chlorine powder or chlorinated lime, calcium hypochlorite is the main active ingredient.
-in calcium hypochlorite, calcium is bonded to two oxygen atoms and oxygen atom is linked to chlorine atom and calcium. Oxidation number of calcium is +2. Oxygen has oxidation state as -2 and chlorine has oxidation state as +1.
-structure of calcium hypochlorite is as follows:
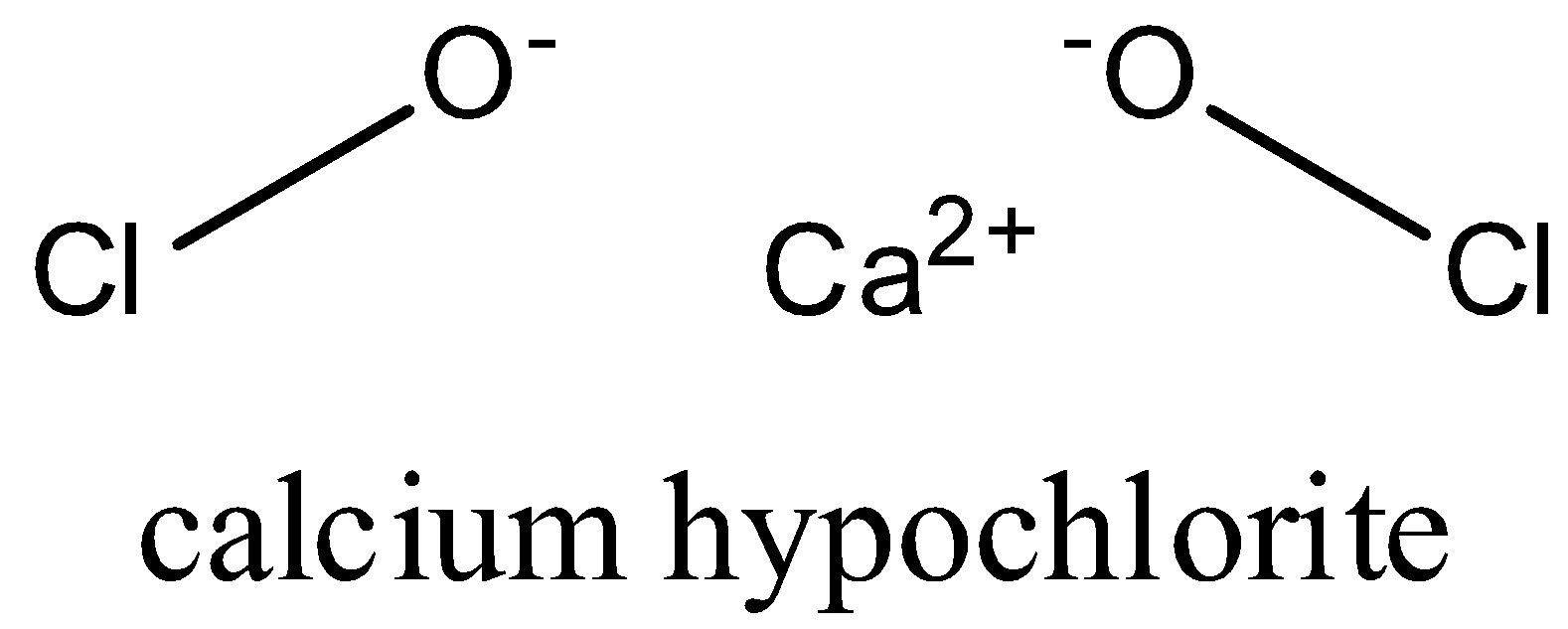
-it is used in water treatment to remove hardness of water and also as a bleaching agent.
-it is an inorganic compound as there is no carbon involved in structure.
- it is more stable and easily available.
-it has a strong smell of chlorine and it decomposes in moist air.
-it has good solubility in soft to medium hard water.
-it is prepared by treating calcium hydroxide known as lime with chlorine gas.
It exists in two forms, dry and hydrated.
-it is commonly used for sanitization of swimming pools. It is a good oxidizing agent.
The chemical formula of calcium hypochlorite is (B) $Ca{{(ClO)}_{2}}$
Note: Calcium hypochlorite is basic in nature. It is used confused with calcium oxychloride. Oxidation number of calcium is +2. Oxygen has oxidation state as -2 and chlorine has oxidation state as +1. It is used as a disinfectant. $COC{{l}_{2}}$ is carbonyl chloride.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




