
The bonds present in \[{{\rm{N}}_2}{{\rm{O}}_5}\] are
A ) only ionic
B ) covalent and coordinate
C ) only covalent
D ) covalent and ionic
Answer
584.4k+ views
Hint: A ionic bond is formed between a metal and non metal. A covalent bond is formed by atoms having similar electronegativities. A coordinate covalent bond is formed when an atom donates both electrons to form a bond.
Complete answer:
Ionic bonds are formed when a metal forms a bond with a non metal. Covalent bonds are formed between two nonmetals.
Both nitrogen and oxygen are non metals. Hence, ionic bonds are not formed between nitrogen and oxygen. Thus, no ionic bond is present in dinitrogen pentoxide molecules. Hence, the option A ) only ionic and the option D ) covalent and ionic are ruled out.
Only two options B ) covalent and coordinate and C ) only covalent are remaining. Out of these two options, one is the correct answer.
The structure of dinitrogen pentoxide is as shown below:
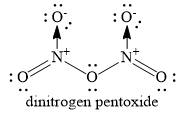
You can see that two coordinate covalent bonds are present. The remaining bonds are covalent bonds. Hence, the option C ) only covalent is ruled out and the correct option is B ) covalent and coordinate.
Thus, dinitrogen pentoxide molecules contain both coordinate bonds and covalent bonds.
So the correct answer is option (B).
Note: Nitrogen atom donates a pair of electrons to oxygen atom to form coordinate covalent bond. Nitrogen atom gains unit positive charge and oxygen atom gains unit negative charge. Similar coordinate covalent bond is formed between the second nitrogen atom and second oxygen atom.
Complete answer:
Ionic bonds are formed when a metal forms a bond with a non metal. Covalent bonds are formed between two nonmetals.
Both nitrogen and oxygen are non metals. Hence, ionic bonds are not formed between nitrogen and oxygen. Thus, no ionic bond is present in dinitrogen pentoxide molecules. Hence, the option A ) only ionic and the option D ) covalent and ionic are ruled out.
Only two options B ) covalent and coordinate and C ) only covalent are remaining. Out of these two options, one is the correct answer.
The structure of dinitrogen pentoxide is as shown below:

You can see that two coordinate covalent bonds are present. The remaining bonds are covalent bonds. Hence, the option C ) only covalent is ruled out and the correct option is B ) covalent and coordinate.
Thus, dinitrogen pentoxide molecules contain both coordinate bonds and covalent bonds.
So the correct answer is option (B).
Note: Nitrogen atom donates a pair of electrons to oxygen atom to form coordinate covalent bond. Nitrogen atom gains unit positive charge and oxygen atom gains unit negative charge. Similar coordinate covalent bond is formed between the second nitrogen atom and second oxygen atom.
Recently Updated Pages
Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 Physics: Engaging Questions & Answers for Success

Master Class 11 Accountancy: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




