
The bondline formula of isopropyl alcohol is as shown above.
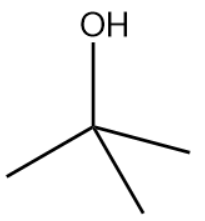
Options-
(A) True
(B) False

Answer
575.7k+ views
Hint: An attempt to this question can be made by determining the rules to be followed by drawing the bondline formula. Now determine the structure of isopropyl alcohol in condensed form. Based on this draw the bondline formula for the same compound, isopropyl alcohol.
Complete answer:
Bond-line structure is often referred to as bond-line formula, skeletal structure, skeletal formula. It is considered as a representation of molecular structure in which covalent bonds are represented with one line for each level of bond order.
A single bond is commonly represented with a single line, a double bond by two parallel lines and triple bond by three parallel lines.
The position of carbon atoms is either shown by letters or may be implied. It is similar to Lewis structure.
The formula for isopropyl alcohol in condensed form is ${{C}_{3}}{{H}_{8}}O$. However, the condensed form does not give a clear idea about the bonding between the atoms. This is the reason why we will now draw the Lewis structure for isopropyl alcohol. The structure is given below:
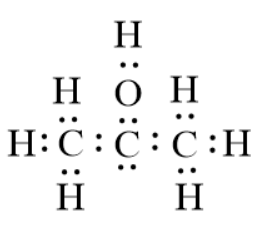
Based on this we will draw the bond-line structure for the compound, isopropyl compound. This can be done by replacing the dots by bond lines.
The bond-line structure is given below:
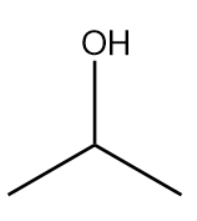
The structure drawn above does not match with the structure given in the question. Therefore, the statement is false.
The correct answer is option (B).
Note:
The IUPAC name for the structure given in the solution is 2-propanol. It is commonly referred to as isopropyl alcohol as well as rubbing alcohol.
Complete answer:
Bond-line structure is often referred to as bond-line formula, skeletal structure, skeletal formula. It is considered as a representation of molecular structure in which covalent bonds are represented with one line for each level of bond order.
A single bond is commonly represented with a single line, a double bond by two parallel lines and triple bond by three parallel lines.
The position of carbon atoms is either shown by letters or may be implied. It is similar to Lewis structure.
The formula for isopropyl alcohol in condensed form is ${{C}_{3}}{{H}_{8}}O$. However, the condensed form does not give a clear idea about the bonding between the atoms. This is the reason why we will now draw the Lewis structure for isopropyl alcohol. The structure is given below:

Based on this we will draw the bond-line structure for the compound, isopropyl compound. This can be done by replacing the dots by bond lines.
The bond-line structure is given below:

The structure drawn above does not match with the structure given in the question. Therefore, the statement is false.
The correct answer is option (B).
Note:
The IUPAC name for the structure given in the solution is 2-propanol. It is commonly referred to as isopropyl alcohol as well as rubbing alcohol.
Recently Updated Pages
Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

How is the angle of emergence e related to the angle class 12 physics CBSE

Differentiate between lanthanoids and actinoids class 12 chemistry CBSE

Derive Lens Makers formula for a convex lens class 12 physics CBSE

a Draw Labelled diagram of Standard Hydrogen Electrode class 12 chemistry CBSE




