
The bond present in$\left( {{{\rm{N}}_{\rm{2}}}{{\rm{O}}_{\rm{5}}}} \right)$ are:
A) Only ionic
B) Covalent
C) Dative
D) Both ionic and covalent
Answer
561k+ views
Hint: We know that the attracting between two atoms or molecules and the formation of new bonds in any chemical species defines the phenomena of chemical bonding. There are mainly two types of bond one is the ionic bond, and another one is the covalent bond.
Complete answer:
As we all know, when two non-metals bonded, they usually formed a covalent bond. In the ${{\rm{N}}_{\rm{2}}}{{\rm{O}}_{\rm{5}}}$ compound, there are two nitrogen atom and five oxygen atom. The name of given chemical formula ${{\rm{N}}_{\rm{2}}}{{\rm{O}}_{\rm{5}}}$ is dinitrogen pentoxide. It is one of the renowned oxides of the nitrogen atom. The atomic number of nitrogen is seven and oxygen is eight.
Generally, the valence electrons of nitrogen are three, and the valence electrons of oxygen are four. They both come under the category of non-metal. Thus, the bond present in the compound ${{\rm{N}}_{\rm{2}}}{{\rm{O}}_{\rm{5}}}$ is four N-O and two N=O. They all are covalent bonds because the sharing of electrons are shown by these atoms. So, the bond present in the ${{\rm{N}}_{\rm{2}}}{{\rm{O}}_{\rm{5}}}$ are covalent bond. Its structure is shown below.
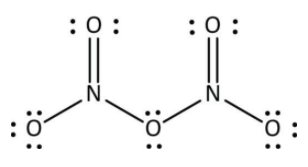
Hence, the correct answer is option ‘B’ i.e, a covalent bond.
Note: In the covalent bonds mainly sharing of electrons of any chemical species takes place. They are commonly termed as molecular bonds also. Electron pairs are known as shared pairs or bonding pairs, and the stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding.
Complete answer:
As we all know, when two non-metals bonded, they usually formed a covalent bond. In the ${{\rm{N}}_{\rm{2}}}{{\rm{O}}_{\rm{5}}}$ compound, there are two nitrogen atom and five oxygen atom. The name of given chemical formula ${{\rm{N}}_{\rm{2}}}{{\rm{O}}_{\rm{5}}}$ is dinitrogen pentoxide. It is one of the renowned oxides of the nitrogen atom. The atomic number of nitrogen is seven and oxygen is eight.
Generally, the valence electrons of nitrogen are three, and the valence electrons of oxygen are four. They both come under the category of non-metal. Thus, the bond present in the compound ${{\rm{N}}_{\rm{2}}}{{\rm{O}}_{\rm{5}}}$ is four N-O and two N=O. They all are covalent bonds because the sharing of electrons are shown by these atoms. So, the bond present in the ${{\rm{N}}_{\rm{2}}}{{\rm{O}}_{\rm{5}}}$ are covalent bond. Its structure is shown below.

Hence, the correct answer is option ‘B’ i.e, a covalent bond.
Note: In the covalent bonds mainly sharing of electrons of any chemical species takes place. They are commonly termed as molecular bonds also. Electron pairs are known as shared pairs or bonding pairs, and the stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE

10 examples of friction in our daily life




