
The bond angles of $N{{H}_{3}},NH_{4}^{+},NH_{2}^{-}$ are in the order:
(1) $NH_{2}^{-}>N{{H}_{3}}>NH_{4}^{+}$
(2) $NH_{4}^{+}>NH_{2}^{-}>N{{H}_{3}}$
(3) $NH_{4}^{+}>N{{H}_{3}}>NH_{2}^{-}$
(4) $N{{H}_{3}}>NH_{4}^{+}>NH_{2}^{-}$
Answer
567k+ views
Hint: To answer this question we should be aware of bond formation, number of lone pairs present on the central atom and its geometry. The number of lone pairs present on the central atom decides the bond angle and geometry.
Complete answer:
-$N{{H}_{3}}$ $N{{H}_{3}}$ has three N-H$\sigma $-bonds each of these are formed by overlap between the singly filled $s{{p}^{3}}$ hybrid on N and 1s orbital of H atom. It has a lone pair of electrons due to which its geometry is tetrahedral not trigonal pyramidal.

The number of lone pairs is 1.
-$NH_{4}^{+}$ $NH_{4}^{+}$ is the ammonium ion. All these ions can be regarded as having been formed by the donation of an electron pair residing on the central atom in $N{{H}_{3}}$ molecules to ${{X}^{+}}$ ions. The formation of $NH_{4}^{+}$, $N{{H}_{3}}$ molecule acts as a Lewis base and ${{X}^{+}}$ acts as Lewis acid. The central atom N is surrounded by four $\sigma $- bonds. So, it is $s{{p}^{3}}$ hybridized and it has no lone pair. Hence, it is tetrahedral.
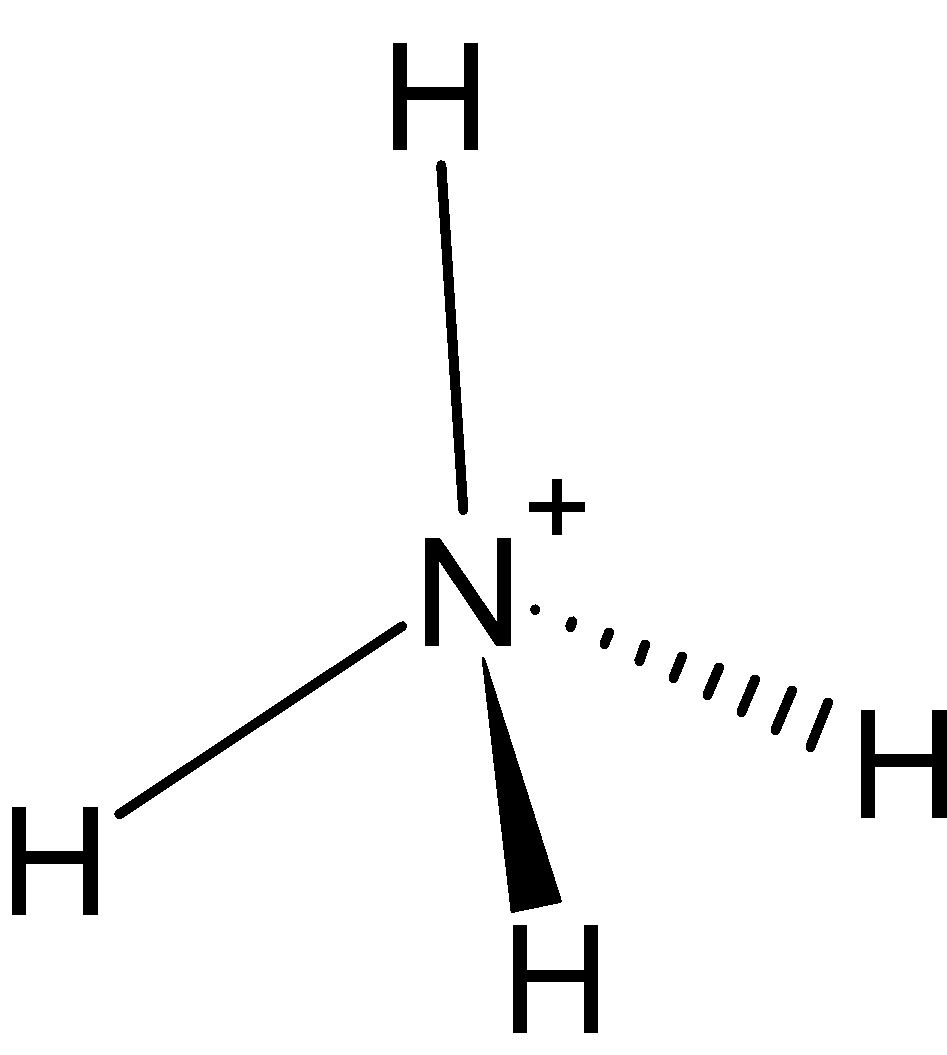
Therefore, the number of lone pairs present on N in $NH_{4}^{+}$ is 0.
- $NH_{2}^{-}$ $NH_{2}^{-}$ is the amide ion. The negative charge on the $NH_{2}^{-}$ ion is supposed to be present on N-atoms. Thus the N atom has 6 electrons in its valence shell. The ${{N}^{-}}$ is $s{{p}^{3}}$ hybridized and it is surrounded by two $\sigma $- bonds and two lone pairs.

The number of lone pairs is 2.
With increase in the number of lone pairs of electrons, the bond angle decreases in the same trend.
Therefore, option 3 is the correct answer.
Note: The number of lone pairs actually decides the bond angle, followed by the geometry of the atom. So, finding out the number of lone pairs of electrons on an N atom, helps in answering this question within no time. $NH_{2}^{-}$ has 2 lone pair, $N{{H}_{3}}$ has one lone pair and $NH_{4}^{+}$ has zero lone pair, therefore option 3 is the correct answer.
Complete answer:
-$N{{H}_{3}}$ $N{{H}_{3}}$ has three N-H$\sigma $-bonds each of these are formed by overlap between the singly filled $s{{p}^{3}}$ hybrid on N and 1s orbital of H atom. It has a lone pair of electrons due to which its geometry is tetrahedral not trigonal pyramidal.

The number of lone pairs is 1.
-$NH_{4}^{+}$ $NH_{4}^{+}$ is the ammonium ion. All these ions can be regarded as having been formed by the donation of an electron pair residing on the central atom in $N{{H}_{3}}$ molecules to ${{X}^{+}}$ ions. The formation of $NH_{4}^{+}$, $N{{H}_{3}}$ molecule acts as a Lewis base and ${{X}^{+}}$ acts as Lewis acid. The central atom N is surrounded by four $\sigma $- bonds. So, it is $s{{p}^{3}}$ hybridized and it has no lone pair. Hence, it is tetrahedral.

Therefore, the number of lone pairs present on N in $NH_{4}^{+}$ is 0.
- $NH_{2}^{-}$ $NH_{2}^{-}$ is the amide ion. The negative charge on the $NH_{2}^{-}$ ion is supposed to be present on N-atoms. Thus the N atom has 6 electrons in its valence shell. The ${{N}^{-}}$ is $s{{p}^{3}}$ hybridized and it is surrounded by two $\sigma $- bonds and two lone pairs.

The number of lone pairs is 2.
With increase in the number of lone pairs of electrons, the bond angle decreases in the same trend.
Therefore, option 3 is the correct answer.
Note: The number of lone pairs actually decides the bond angle, followed by the geometry of the atom. So, finding out the number of lone pairs of electrons on an N atom, helps in answering this question within no time. $NH_{2}^{-}$ has 2 lone pair, $N{{H}_{3}}$ has one lone pair and $NH_{4}^{+}$ has zero lone pair, therefore option 3 is the correct answer.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




