
The Birch reduction of toluene gives:
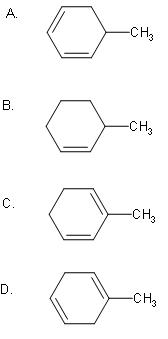

Answer
594k+ views
Hint: For identifying the correct product of the toluene firstly we have to study about the Birch reduction and then we have to write the whole reaction of toluene (Benzene with methyl group) that undergoes Birch reduction.
Complete step by step answer:
- In the given questions, we have to identify the correct product which is formed when the toluene undergoes Birch reduction.
- As we know that the reduction is the process in which the addition of the hydrogen atom and removal of the oxygen atom takes place.
- In the Birch reduction, the aromatic ring the methyl group is also known as toluene, in it the double bond breaks and the attachment of hydrogen take place.
- The chemical reaction will be:
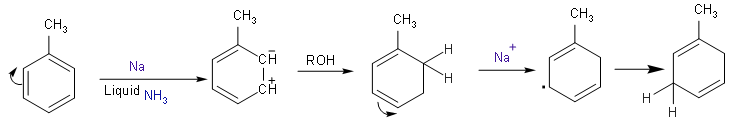
- Here, the reduction is also known as 1, 4 reductions because the reduction or addition of hydrogen at the first and fourth carbon atom takes place.
- The reaction takes place in the presence of sodium or lithium metal and liquid ammonia in the presence of an alcohol such as ethanol.
- At the end as we can see that the formation of unconjugated cyclohexadiene takes place because the single and double bonds are not present in an alternate manner.
So, the correct answer is “Option D”.
Note: While reducing the compound with the Birch reduction method the temperature is usually kept low so that the liquid ammonia can be used as a solvent. The method is very useful when only one double bond is required to break.
Complete step by step answer:
- In the given questions, we have to identify the correct product which is formed when the toluene undergoes Birch reduction.
- As we know that the reduction is the process in which the addition of the hydrogen atom and removal of the oxygen atom takes place.
- In the Birch reduction, the aromatic ring the methyl group is also known as toluene, in it the double bond breaks and the attachment of hydrogen take place.
- The chemical reaction will be:

- Here, the reduction is also known as 1, 4 reductions because the reduction or addition of hydrogen at the first and fourth carbon atom takes place.
- The reaction takes place in the presence of sodium or lithium metal and liquid ammonia in the presence of an alcohol such as ethanol.
- At the end as we can see that the formation of unconjugated cyclohexadiene takes place because the single and double bonds are not present in an alternate manner.
So, the correct answer is “Option D”.
Note: While reducing the compound with the Birch reduction method the temperature is usually kept low so that the liquid ammonia can be used as a solvent. The method is very useful when only one double bond is required to break.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Organisms of a higher trophic level which feed on several class 12 biology CBSE

Give 10 examples of unisexual and bisexual flowers

Give simple chemical tests to distinguish between the class 12 chemistry CBSE




