
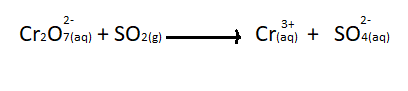
What will be the balanced equation in acidic medium for the given reaction ?
$C{r_2}{O^{2 - }}_{7(aq)} + S{O_{2(g)}} \to C{r^{3 + }}_{(aq)} + S{O_4}{^{2 - }_{(aq)}}$
A. $C{r_2}{O^{2 - }}_{7(aq)} + 3S{O_{2(g)}} + 2{H^ + }_{\left( {aq} \right)} \to 2C{r^{3 + }}_{(aq)} + 3S{O_4}{^{2 - }_{(aq)}} + {H_2}{O_{(l)}}$
B. $2C{r_2}{O^{2 - }}_{7(aq)} + 3S{O_{2(g)}} + 4{H^ + }_{\left( {aq} \right)} \to 4C{r^{3 + }}_{(aq)} + 3S{O_4}{^{2 - }_{(aq)}} + 2{H_2}{O_{(l)}}$
C. $C{r_2}{O^{2 - }}_{7(aq)} + 3S{O_{2(g)}} + 14{H^ + }_{\left( {aq} \right)} \to 2C{r^{3 + }}_{(aq)} + 3S{O_4}{^{2 - }_{(aq)}} + 7{H_2}{O_{(l)}}$
D. $C{r_2}{O^{2 - }}_{7(aq)} + 6S{O_{2(g)}} + 7{H^ + }_{\left( {aq} \right)} \to 2C{r^{3 + }}_{(aq)} + 6S{O_4}{^{2 - }_{(aq)}} + 7{H_2}{O_{(l)}}$
Answer
598.2k+ views
Hint: To balance a redox reaction is more complex than balancing standard reactions. The method that is used to balance redox reaction is called the half equation method. These acidic equations are balanced by adding ${H_2}O,{H^ + }$ and ${e^ - }$.There is a rule to balance redox reaction which is as follows:
Firstly we have to write separate half reactions then we should balance all the elements in the equation except oxygen and hydrogen.
Then balance the equation by addition of an appropriate number of water molecules to the opposite side of the reaction.
In step 2 we had added a water molecule then we have to balance hydrogen atoms here so we will balance the hydrogen atom by adding ${H^ + }$ ion to the opposite side of the equation where the water molecule is added.
Now we have to balance charge in the equation so we will add electrons at that side which is more positive. Mostly ${H^ + }$ ion and ${e^ - }$ are always on the same side.
Then we will balance electrons to each side by multiplying it to the appropriate integers.
Then half equations are added together. Then we cancel out the electron to form a balanced equation.
Complete answer:
Now we will write half equations here; Equation (i) is oxidation reaction and Equation (ii) is reduction reaction,
$S{O_2} \to S{O^{2 - }}_4$ ……..(i) $C{r_2}{O^{ - 2}}_7 \to C{r^{ + 3}}$ ……..(ii)
$ \Rightarrow S{O_2} + 2{H_2}O \to S{O^{2 - }}_4$ $ \Rightarrow C{r_2}{O^{ - 2}}_7 \to 2C{r^{ + 3}}$
$ \Rightarrow S{O_2} + 2{H_2}O \to S{O^{2 - }}_4 + 4{H^ + }$ $ \Rightarrow C{r_2}{O^{ - 2}}_7 \to 2C{r^{ + 3}} + 7{H_2}O$
$ \Rightarrow S{O_2} + 2{H_2}O \to S{O^{2 - }}_4 + 4{H^ + } + 2{e^ - }$ $ \Rightarrow C{r_2}{O^{ - 2}}_7 + 14{H^ + } \to 2C{r^{ + 3}} + 7{H_2}O$
$ \Rightarrow (S{O_2} + 2{H_2}O \to S{O^{2 - }}_4 + 4{H^ + } + 2{e^ - }) \times 3$ ……………...(iii) $ \Rightarrow C{r_2}{O^{ - 2}}_7 + 14{H^ + } + 6{e^ - } \to 2C{r^{ + 3}} + 7{H_2}O$ …………(iv)
Now add equation (iii) and (iv) and cancel out the electron to form balance equation,
$3S{O_2} + 2H{3_2}O \to 3S{O^{2 - }}_4 + 12{H^ + } + 6{e^ - }$
$C{r_2}{O^{ - 2}}_7 + 14{H^ + } + 6{e^ - } \to 2C{r^{ + 3}} + 7{H_2}O$
________________________________________________
$C{r_2}{O^{ - 2}}_7 + 3S{O_2} + 2{H^ + } \to 2C{r^{ + 3}} + 3S{O_4}^{ - 2} + {H_2}O$ ………….(v)
So the equation (v) is the balanced equation and here option A is correct .
Note: So we have learned the half reaction method to balance a redox reaction. We should remember this method as it is much easier than any method .Here in the equation (v) we can see every element is balanced and even charges are also balanced.
Firstly we have to write separate half reactions then we should balance all the elements in the equation except oxygen and hydrogen.
Then balance the equation by addition of an appropriate number of water molecules to the opposite side of the reaction.
In step 2 we had added a water molecule then we have to balance hydrogen atoms here so we will balance the hydrogen atom by adding ${H^ + }$ ion to the opposite side of the equation where the water molecule is added.
Now we have to balance charge in the equation so we will add electrons at that side which is more positive. Mostly ${H^ + }$ ion and ${e^ - }$ are always on the same side.
Then we will balance electrons to each side by multiplying it to the appropriate integers.
Then half equations are added together. Then we cancel out the electron to form a balanced equation.
Complete answer:

Now we will write half equations here; Equation (i) is oxidation reaction and Equation (ii) is reduction reaction,
$S{O_2} \to S{O^{2 - }}_4$ ……..(i) $C{r_2}{O^{ - 2}}_7 \to C{r^{ + 3}}$ ……..(ii)
$ \Rightarrow S{O_2} + 2{H_2}O \to S{O^{2 - }}_4$ $ \Rightarrow C{r_2}{O^{ - 2}}_7 \to 2C{r^{ + 3}}$
$ \Rightarrow S{O_2} + 2{H_2}O \to S{O^{2 - }}_4 + 4{H^ + }$ $ \Rightarrow C{r_2}{O^{ - 2}}_7 \to 2C{r^{ + 3}} + 7{H_2}O$
$ \Rightarrow S{O_2} + 2{H_2}O \to S{O^{2 - }}_4 + 4{H^ + } + 2{e^ - }$ $ \Rightarrow C{r_2}{O^{ - 2}}_7 + 14{H^ + } \to 2C{r^{ + 3}} + 7{H_2}O$
$ \Rightarrow (S{O_2} + 2{H_2}O \to S{O^{2 - }}_4 + 4{H^ + } + 2{e^ - }) \times 3$ ……………...(iii) $ \Rightarrow C{r_2}{O^{ - 2}}_7 + 14{H^ + } + 6{e^ - } \to 2C{r^{ + 3}} + 7{H_2}O$ …………(iv)
Now add equation (iii) and (iv) and cancel out the electron to form balance equation,
$3S{O_2} + 2H{3_2}O \to 3S{O^{2 - }}_4 + 12{H^ + } + 6{e^ - }$
$C{r_2}{O^{ - 2}}_7 + 14{H^ + } + 6{e^ - } \to 2C{r^{ + 3}} + 7{H_2}O$
________________________________________________
$C{r_2}{O^{ - 2}}_7 + 3S{O_2} + 2{H^ + } \to 2C{r^{ + 3}} + 3S{O_4}^{ - 2} + {H_2}O$ ………….(v)
So the equation (v) is the balanced equation and here option A is correct .
Note: So we have learned the half reaction method to balance a redox reaction. We should remember this method as it is much easier than any method .Here in the equation (v) we can see every element is balanced and even charges are also balanced.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




