
The average charge on each o atom and average bond order of I-O bond in $\text{I}{{\text{O}}_{6}}^{5-}$ is
(A) $-1$ and $1\cdot 67$
(B) $-5/6$ and $1\cdot 33$
(C) $-5/6$ and $1\cdot 67$
(D) $-5/6$ and $1\cdot 167$
Answer
560.7k+ views
Hint $\text{I}{{\text{O}}_{6}}^{5-}$: I is central atom where I surrounds by the six oxygen atom with negative charge. In this structure we have to calculate average charge and average bond order.
Average charge$=\dfrac{\text{Total charge of molecule}}{\text{No}\text{. of atom responsible for charge}}$
Bond order$=\dfrac{\text{Total bonds}}{\text{Total atom surrounding}}$
Complete step by step solution:
In$\text{I}{{\text{O}}_{6}}^{5-}$ the structure as follows
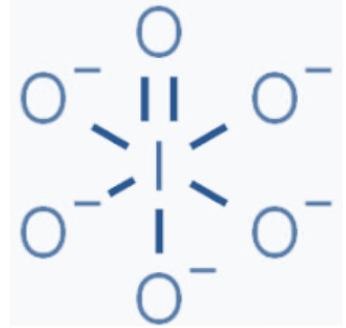
Charge on each oxygen atom=1
Total charge on molecule contributed by oxygen atom=$-5$
Average charge$=\dfrac{\text{Charge on molecule }}{\text{No}\text{. of total oxygen atom}}$
$\implies\dfrac{-5}{6}$
Average bond order:
No. of total bonds=7 [as one is double bond and 5 is single bond]
Total no. of oxygen
$\begin{align}
&\implies \dfrac{7}{6} \\
&\implies 1\cdot 16 \\
\end{align}$
So the correct option is ‘D’
Note: Covalent bond: A bond which is formed by the shared pair of electrons is called covalent bond. Further covalent bond divided into two kinds.
Sigma Bond: A bond which is formed by overlapping of the internuclear axis is called sigma bond. It is of three kinds S-S overlapping, S-P overlapping, P-P overlapping.
Pi-Bond: A bond which is formed by the sideways overlapping of P-orbital is called pi-bond
Average charge$=\dfrac{\text{Total charge of molecule}}{\text{No}\text{. of atom responsible for charge}}$
Bond order$=\dfrac{\text{Total bonds}}{\text{Total atom surrounding}}$
Complete step by step solution:
In$\text{I}{{\text{O}}_{6}}^{5-}$ the structure as follows

Charge on each oxygen atom=1
Total charge on molecule contributed by oxygen atom=$-5$
Average charge$=\dfrac{\text{Charge on molecule }}{\text{No}\text{. of total oxygen atom}}$
$\implies\dfrac{-5}{6}$
Average bond order:
No. of total bonds=7 [as one is double bond and 5 is single bond]
Total no. of oxygen
$\begin{align}
&\implies \dfrac{7}{6} \\
&\implies 1\cdot 16 \\
\end{align}$
So the correct option is ‘D’
Note: Covalent bond: A bond which is formed by the shared pair of electrons is called covalent bond. Further covalent bond divided into two kinds.
Sigma Bond: A bond which is formed by overlapping of the internuclear axis is called sigma bond. It is of three kinds S-S overlapping, S-P overlapping, P-P overlapping.
Pi-Bond: A bond which is formed by the sideways overlapping of P-orbital is called pi-bond
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




