
The addition of HBr is easiest with:
(A) $C{{H}_{2}}=CH-Cl$
(B) $Cl-CH=CH-Cl$
(C) $C{{H}_{3}}-CH=C{{H}_{2}}$
(D) ${{(C{{H}_{3}})}_{2}}C=C{{H}_{2}}$
Answer
578.7k+ views
Hint: Stability of carbocation increases by the presence of electron donating groups around and more the number of alpha hydrogen atoms, more is the stability of carbocation, this undergoes by the process of hyperconjugation.
Complete answer:
Addition of hydrogen halide to alkene proceed through formation of carbocation.
A carbocation is a molecule in which a carbon atom has a positive charge and three bonds. We can basically say that they are carbon cations. Formerly, it was known as carbonium ion. Carbocation today is defined as any even-electron cation that possesses a significant positive charge on the carbon atom.
Carbocation gets stabilised by hyperconjugation and inductive effect, Increasing substitution, increases the hyperconjugation and thus it increases stability. More the hyperconjugation more is the stability.
\[{{R}_{3}}{{C}^{+~}}\left( {{3}^{o}}~;\text{ }most\text{ }stable \right)\text{ }>\text{ }{{R}_{2}}C{{H}^{+}}~\left( {{2}^{o}}~ \right)\text{ }>\text{ }RC{{H}_{2}}^{+}~\left( {{1}^{o}} \right)\text{ }C{{H}_{3}}^{+}~\left( methyl;\text{ }least\text{ }stable \right)\]
Option A and B have Chlorine present in the compound, which is an electron withdrawing group and it destabilizes the carbocation formed.
Considering option C, the carbocation formed will be of secondary carbon and will have 6 alpha hydrogens. The structure of intermediate will be as follows:
\[C{{H}_{3}}-C{{H}^{+}}-C{{H}_{3}}\]
Considering option D, the carbocation formed will be of tertiary carbon and will have 9 alpha hydrogens. The structure of intermediate will be as follows:
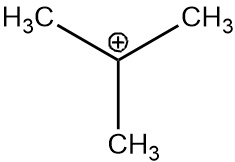
Therefore, the addition of HBr will be easiest with the D option.
Note:
Hyperconjugation is the stabilising interaction that results from the interaction of the electrons in a $\sigma $ -bond (usually C-H or C-C) with an adjacent empty or partially filled p-orbital or a $\pi $ -orbital to give an extended molecular orbital that increases the stability of the system.
Complete answer:
Addition of hydrogen halide to alkene proceed through formation of carbocation.
A carbocation is a molecule in which a carbon atom has a positive charge and three bonds. We can basically say that they are carbon cations. Formerly, it was known as carbonium ion. Carbocation today is defined as any even-electron cation that possesses a significant positive charge on the carbon atom.
Carbocation gets stabilised by hyperconjugation and inductive effect, Increasing substitution, increases the hyperconjugation and thus it increases stability. More the hyperconjugation more is the stability.
\[{{R}_{3}}{{C}^{+~}}\left( {{3}^{o}}~;\text{ }most\text{ }stable \right)\text{ }>\text{ }{{R}_{2}}C{{H}^{+}}~\left( {{2}^{o}}~ \right)\text{ }>\text{ }RC{{H}_{2}}^{+}~\left( {{1}^{o}} \right)\text{ }C{{H}_{3}}^{+}~\left( methyl;\text{ }least\text{ }stable \right)\]
Option A and B have Chlorine present in the compound, which is an electron withdrawing group and it destabilizes the carbocation formed.
Considering option C, the carbocation formed will be of secondary carbon and will have 6 alpha hydrogens. The structure of intermediate will be as follows:
\[C{{H}_{3}}-C{{H}^{+}}-C{{H}_{3}}\]
Considering option D, the carbocation formed will be of tertiary carbon and will have 9 alpha hydrogens. The structure of intermediate will be as follows:

Therefore, the addition of HBr will be easiest with the D option.
Note:
Hyperconjugation is the stabilising interaction that results from the interaction of the electrons in a $\sigma $ -bond (usually C-H or C-C) with an adjacent empty or partially filled p-orbital or a $\pi $ -orbital to give an extended molecular orbital that increases the stability of the system.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




