
The absolute configuration of the following compound is:
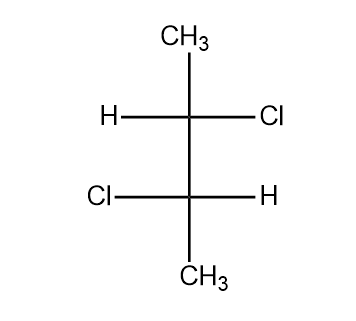
(a)$2S,3R$
(b)$2S,3S$
(c)$2R,3S$
(d)$2R,3R$

Answer
508.2k+ views
Hint: An absolute configuration is the spatial arrangement of a chiral molecule and its stereochemical description that is R and S. R means Rectus and S means Sinister. These absolute configurations are mostly obtained by X-ray crystallography with some limitations also.
Complete answer:
If we rotate the curve in clockwise direction, the absolute configuration we get is R and if we rotate the curve in anti-clockwise direction, the absolute configuration we get is S. But some limitation is there. The lowest priority group should be present at the vertical position, otherwise the absolute configuration gets reversed. So from the above image, we see that H is the least priority group and $Cl$ is the highest priority group. So, we start from chlorine after that moves to the third carbon and then to the methyl group and finally to the hydrogen. We can see that this rotation is in clockwise direction but H is in horizontal position. So the absolute configuration for the $2$ carbon is $2S$. Similarly, if we look for third carbon, again the highest priority group is $Cl$. So we start from $Cl$ and move to the second carbon and then the methyl group. But H is present in the horizontal direction. So the absolute configuration is $3S$. So the absolute configuration of the compound is $2S,3S$.
Therefore, option B is the correct option.
Note:
There are also other configurations present in the stereochemistry which are D and L. D stands for dextro-rotatory and L is called levo-rotatory. Dextro-rotatory means the rotation is + means in clockwise direction and levo-rotatory means the direction is – means in anti-clockwise direction.
Complete answer:
If we rotate the curve in clockwise direction, the absolute configuration we get is R and if we rotate the curve in anti-clockwise direction, the absolute configuration we get is S. But some limitation is there. The lowest priority group should be present at the vertical position, otherwise the absolute configuration gets reversed. So from the above image, we see that H is the least priority group and $Cl$ is the highest priority group. So, we start from chlorine after that moves to the third carbon and then to the methyl group and finally to the hydrogen. We can see that this rotation is in clockwise direction but H is in horizontal position. So the absolute configuration for the $2$ carbon is $2S$. Similarly, if we look for third carbon, again the highest priority group is $Cl$. So we start from $Cl$ and move to the second carbon and then the methyl group. But H is present in the horizontal direction. So the absolute configuration is $3S$. So the absolute configuration of the compound is $2S,3S$.
Therefore, option B is the correct option.
Note:
There are also other configurations present in the stereochemistry which are D and L. D stands for dextro-rotatory and L is called levo-rotatory. Dextro-rotatory means the rotation is + means in clockwise direction and levo-rotatory means the direction is – means in anti-clockwise direction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Give 10 examples of unisexual and bisexual flowers




