
The above shown polymer is obtained when a carbon compound is allowed to stand. It is a white solid. The polymer is:
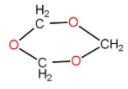
A.Trioxane
B.Formose
C.Paraformaldehyde
D.Metaldehyde

Answer
579k+ views
Hint: The polymer contains three oxygen groups and it is used with formaldehyde and paraformaldehyde. It is also used in the production of polyoxymethylene plastics.
Complete step by step answer:
1,3,5 trioxane is also known as trioxane, is a compound with the structure as follow:
It has a chloroform like smell and is a white solid. It exists in three isomers. Basically the formation of trioxane is done by acid catalysed cyclic trimerization of formaldehyde with concentrated sulfuric acid. Three moles of formaldehyde form trioxane. The water molecule reacted and generated back with the product. So basically a carbon compound that is formaldehyde is getting converted to tricyclic compound. The reaction occurs as follow:
\[3{{\text{H}}_2}{\text{CO}} + 3{{\text{H}}_2}{\text{O}} \rightleftharpoons {{\text{C}}_3}{{\text{H}}_6}{{\text{O}}_3} + 3{{\text{H}}_2}{\text{O}}\]
Hence, the correct option is A.
Additional Information:
Formose is a naming reaction that was discovered by Aleksandr butlerov. Paraformaldehyde is produced by the polymerization of formaldehyde. Metaldehyde is an organic compound with the molecular formula \[{\text{C}}{{\text{H}}_3}{\text{CH}}{{\text{O}}_4}\]. It is used as a pesticide and it is a cyclic trimer of acetaldehyde.
Note:
Formaldehyde is a simple chemical compound made of hydrogen and oxygen. It is the simplest aldehyde. In pure state it occurs as a gas and is pungent smelling and colourless gas. The formaldehyde gas polymerises into paraformaldehyde gas readily. It is used for the synthesis of many complex compounds including urea formaldehyde resin, phenol formaldehyde resin, diphenyl isocyanide. It is also used as disinfectant as it kills many bacteria and fungi, when it is in aqueous solution.
Complete step by step answer:
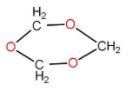
1,3,5 trioxane is also known as trioxane, is a compound with the structure as follow:

It has a chloroform like smell and is a white solid. It exists in three isomers. Basically the formation of trioxane is done by acid catalysed cyclic trimerization of formaldehyde with concentrated sulfuric acid. Three moles of formaldehyde form trioxane. The water molecule reacted and generated back with the product. So basically a carbon compound that is formaldehyde is getting converted to tricyclic compound. The reaction occurs as follow:
\[3{{\text{H}}_2}{\text{CO}} + 3{{\text{H}}_2}{\text{O}} \rightleftharpoons {{\text{C}}_3}{{\text{H}}_6}{{\text{O}}_3} + 3{{\text{H}}_2}{\text{O}}\]
Hence, the correct option is A.
Additional Information:
Formose is a naming reaction that was discovered by Aleksandr butlerov. Paraformaldehyde is produced by the polymerization of formaldehyde. Metaldehyde is an organic compound with the molecular formula \[{\text{C}}{{\text{H}}_3}{\text{CH}}{{\text{O}}_4}\]. It is used as a pesticide and it is a cyclic trimer of acetaldehyde.
Note:
Formaldehyde is a simple chemical compound made of hydrogen and oxygen. It is the simplest aldehyde. In pure state it occurs as a gas and is pungent smelling and colourless gas. The formaldehyde gas polymerises into paraformaldehyde gas readily. It is used for the synthesis of many complex compounds including urea formaldehyde resin, phenol formaldehyde resin, diphenyl isocyanide. It is also used as disinfectant as it kills many bacteria and fungi, when it is in aqueous solution.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




