
How can you tell which atoms are in the same plane of cumulene? Why is allene preferentially twisted instead of planar?
Answer
494.4k+ views
Hint: Cumulenes are chemical compounds which are formed by carbon and hydrogen atoms with three or more consecutive double bonds. Allenes are formed with carbon atoms having propadiene as their parent compound.
Complete answer:
Cumulene structure is rigid in nature due to the formation of double bonds between the carbon atoms. All the carbon atoms are $ sp $ hybridized in the structure which forms the double bond in cumulene. This $ sp $ hybridization results in formation of two $ \left( \pi \right) $ bonds, one with each neighbor carbon atom. These double bonds are perpendicular in nature with respect to carbon atoms and therefore leads to the formation of a linear geometry within the carbon chain.
Example of the simplest cumulene is butatriene with three consecutive double bonds.
Structure of cumulene is- butadiene

From the structure we clearly see that the entire structure of cumulene is present on the same plane.
In the allene the center carbon atom is associated with formation of two sigma bonds and two pi bonds. Hybridization of carbon atoms is $ sp $ while the other two terminal carbon atoms are $ s{p^2} $ hybridized. The bond angle is $ {180^ \circ } $ which is formed by the three carbon atoms. This confers linear geometry but the terminal two carbon atoms have planar geometry but the bond angle is twisted $ {90^ \circ } $ from each other.
Structure of allene is - $ 1,2 - $ propadiene
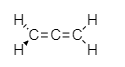
From the structure we clearly see that terminal hydrogen is twisted and out of the plane.
$ \Rightarrow $ From the above discussion we conclude that cumulene is a planar molecule while allene is a twisted molecule.
Note:
Carbon suboxide is an example of cumulene. Cumulene are considered as cis-trans depending upon the number of double bonds. Cis-trans cumulene has odd number of double bonds in the compound. Allenes have a tendency to undergo the cyclo-addition reaction.
Complete answer:
Cumulene structure is rigid in nature due to the formation of double bonds between the carbon atoms. All the carbon atoms are $ sp $ hybridized in the structure which forms the double bond in cumulene. This $ sp $ hybridization results in formation of two $ \left( \pi \right) $ bonds, one with each neighbor carbon atom. These double bonds are perpendicular in nature with respect to carbon atoms and therefore leads to the formation of a linear geometry within the carbon chain.
Example of the simplest cumulene is butatriene with three consecutive double bonds.
Structure of cumulene is- butadiene

From the structure we clearly see that the entire structure of cumulene is present on the same plane.
In the allene the center carbon atom is associated with formation of two sigma bonds and two pi bonds. Hybridization of carbon atoms is $ sp $ while the other two terminal carbon atoms are $ s{p^2} $ hybridized. The bond angle is $ {180^ \circ } $ which is formed by the three carbon atoms. This confers linear geometry but the terminal two carbon atoms have planar geometry but the bond angle is twisted $ {90^ \circ } $ from each other.
Structure of allene is - $ 1,2 - $ propadiene
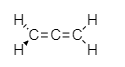
From the structure we clearly see that terminal hydrogen is twisted and out of the plane.
$ \Rightarrow $ From the above discussion we conclude that cumulene is a planar molecule while allene is a twisted molecule.
Note:
Carbon suboxide is an example of cumulene. Cumulene are considered as cis-trans depending upon the number of double bonds. Cis-trans cumulene has odd number of double bonds in the compound. Allenes have a tendency to undergo the cyclo-addition reaction.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




