
TEL, tetraethyl lead, acts as an anti-knocking agent. It acts as:
A.Negative catalyst
B.Catalyst
C.Electrophile
D.Nucleophile
Answer
588k+ views
Hint: We have to know that tetraethyl lead is an example of organolead compound. The chemical formula of TEL is ${\left( {C{H_3}C{H_2}} \right)_4}Pb.$ we can write the IUPAC name of TEL as Tetraethylplumbane. It appears as colourless liquid and has pleasant and sweet odour.
Complete step by step answer:
We can synthesize TEL from chloroethane and sodium-lead alloy. We can write the chemical equation is,
$4NaPb + 4C{H_3}C{H_2}Cl\xrightarrow{{}}{\left( {C{H_3}C{H_2}} \right)_4}Pb + 4NaCl + 3Pb$
We can obtain the product TEL by steam distillation, the byproducts obtained are lead and sodium chloride.
We have to know that TEL is viscous liquid. The charge on TEL is neutral and comprises exterior alkyl groups, due to which it is highly lipophilic and is soluble in gasoline.
We can draw the structure of TEL as,
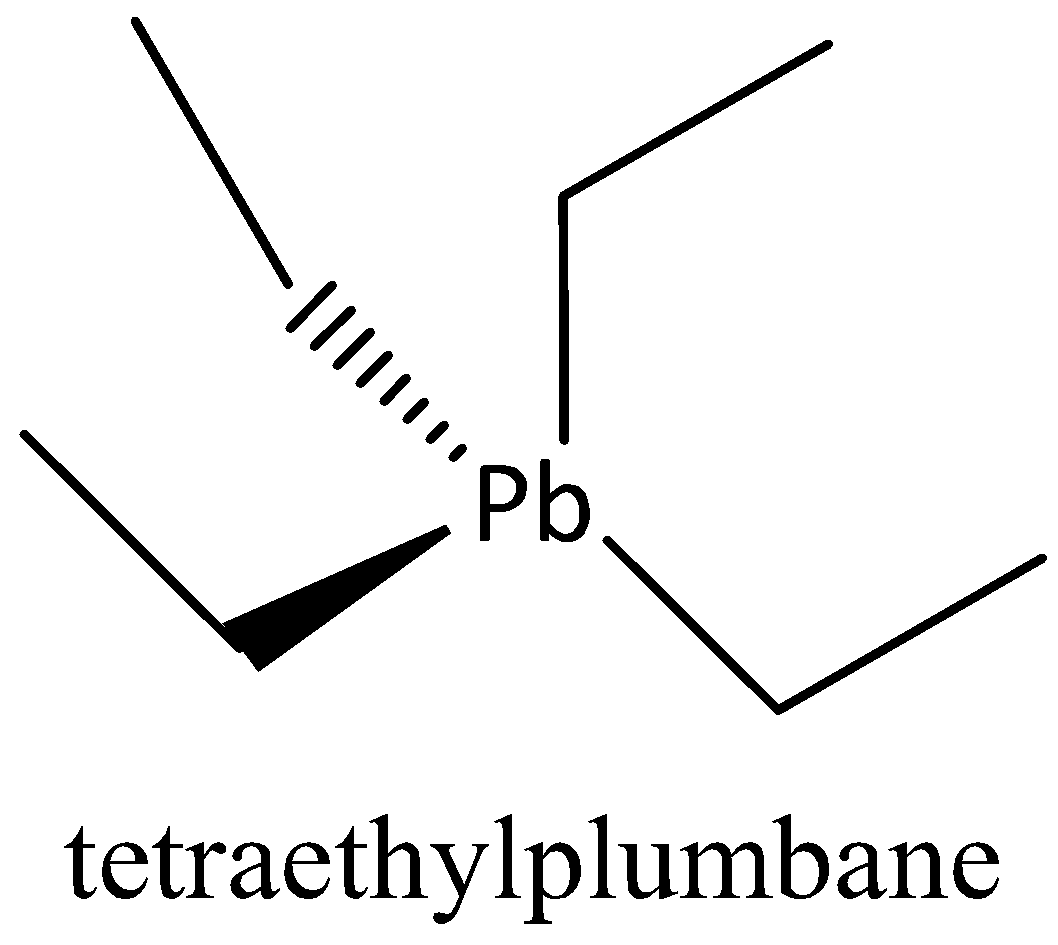
We can see that a single atom of lead is linked to four ethyl groups through an atom a carbon. It adopts a tetrahedral structure.
When we inhale or absorb TEL through the skin, it could cause acute (or) chronic lead poisoning. A few other names of tetraethyl lead are lead tetraethyl, tetra-ethyl lead.
Tetraethyl lead is insoluble in water and has flash point of ${163^ \circ }F.$ The density of TEL is $14\,lb/gal.$
TEL functions as a negative catalyst. Anti-knocking substance is added to petrol to reduce the ignition of vapors of petrol is TEL.
Hence, the correct option is option A.
Note:
We know that TEL is a petrol-fuel additive that was first mixed with gasoline. Countries such as Algeria, Iraq and Yemen use leaded engine gasoline for automobiles. Some of the related compounds to tetraethyl lead are tetraethyl methane, tetraethyl tin and tetramethyl germanium.
Complete step by step answer:
We can synthesize TEL from chloroethane and sodium-lead alloy. We can write the chemical equation is,
$4NaPb + 4C{H_3}C{H_2}Cl\xrightarrow{{}}{\left( {C{H_3}C{H_2}} \right)_4}Pb + 4NaCl + 3Pb$
We can obtain the product TEL by steam distillation, the byproducts obtained are lead and sodium chloride.
We have to know that TEL is viscous liquid. The charge on TEL is neutral and comprises exterior alkyl groups, due to which it is highly lipophilic and is soluble in gasoline.
We can draw the structure of TEL as,

We can see that a single atom of lead is linked to four ethyl groups through an atom a carbon. It adopts a tetrahedral structure.
When we inhale or absorb TEL through the skin, it could cause acute (or) chronic lead poisoning. A few other names of tetraethyl lead are lead tetraethyl, tetra-ethyl lead.
Tetraethyl lead is insoluble in water and has flash point of ${163^ \circ }F.$ The density of TEL is $14\,lb/gal.$
TEL functions as a negative catalyst. Anti-knocking substance is added to petrol to reduce the ignition of vapors of petrol is TEL.
Hence, the correct option is option A.
Note:
We know that TEL is a petrol-fuel additive that was first mixed with gasoline. Countries such as Algeria, Iraq and Yemen use leaded engine gasoline for automobiles. Some of the related compounds to tetraethyl lead are tetraethyl methane, tetraethyl tin and tetramethyl germanium.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




