
What is the systematic name of \[Mg{\left( {N{O_3}} \right)_2}\] ?
Answer
533.7k+ views
Hint: Systematic name is most commonly known as IUPAC name. IUPAC stands for International Union of Pure and Applied Chemistry. It is the world authority on the chemical nomenclature as well as terminology. In this question we have to tell the systematic name of \[Mg{\left( {N{O_3}} \right)_2}\] which is an inorganic compound used in the synthesis of concentrated nitric acid.
Complete step by step answer:
IUPAC provides consistency to the names of organic compounds. It enables every compound to possess a unique name, which otherwise is not plausible with the common names. The chemical structure for \[Mg{\left( {N{O_3}} \right)_2}\] is shown below:
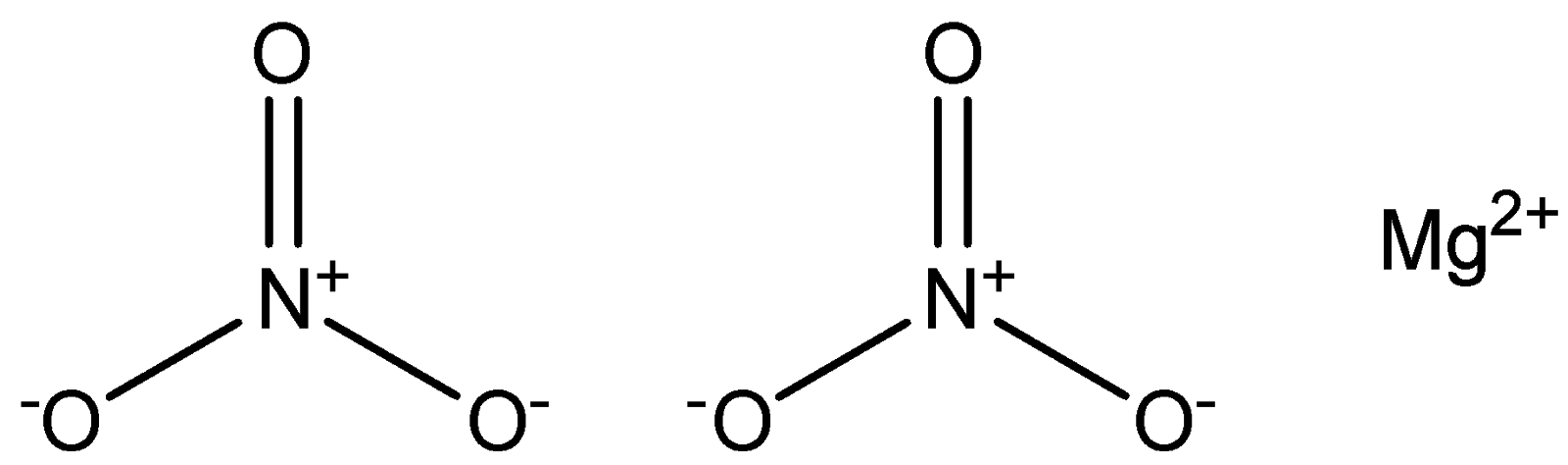
As clear from its chemical formula and structure, \[Mg{\left( {N{O_3}} \right)_2}\] comprises a metal and a group of nonmetals. We know that any compound containing a metal and a non-metal is considered to be an ionic compound. Now let us identify the systematic name for the given compound i.e. \[Mg{\left( {N{O_3}} \right)_2}\] . \[Mg{\left( {N{O_3}} \right)_2}\] consist of two polyatomic ions (i.e. group of nonmetals ( \[{\left( {N{O_3}} \right)_2}\] in this case) after the metal i.e. \[Mg\] in this case) so we'll use a table of names for the common polyatomic ions, along with the Periodic Table. Rules to write the systematic name of Ternary Ionic Compounds are listed below:
1) Name the metal (i.e. cation) according to its appearance in the Periodic Table. For example: \[N{a^ + } = Sodium,{\text{ }}M{g^{2 + }} = Magnesium,{\text{ }}A{l^{3 + }} = Aluminum\]
In the present case, \[Mg\] stands for Magnesium.
2) Find the name of the polyatomic ion according to its appearance in the Common Ion Table.
In the present case, \[N{O_3}\] stands for nitrate.
Hence after combining the name of metal and polyatomic ion, the systematic name of \[Mg{\left( {N{O_3}} \right)_2}\] is Magnesium nitrate.
Note: If you are given the systematic name of an ionic compound and you are asked to find the chemical formula of that compound, take into account the charges of each of the elements present in the compound. Make sure that the charges are balanced such that net charge for the compound must be zero.
Complete step by step answer:
IUPAC provides consistency to the names of organic compounds. It enables every compound to possess a unique name, which otherwise is not plausible with the common names. The chemical structure for \[Mg{\left( {N{O_3}} \right)_2}\] is shown below:

As clear from its chemical formula and structure, \[Mg{\left( {N{O_3}} \right)_2}\] comprises a metal and a group of nonmetals. We know that any compound containing a metal and a non-metal is considered to be an ionic compound. Now let us identify the systematic name for the given compound i.e. \[Mg{\left( {N{O_3}} \right)_2}\] . \[Mg{\left( {N{O_3}} \right)_2}\] consist of two polyatomic ions (i.e. group of nonmetals ( \[{\left( {N{O_3}} \right)_2}\] in this case) after the metal i.e. \[Mg\] in this case) so we'll use a table of names for the common polyatomic ions, along with the Periodic Table. Rules to write the systematic name of Ternary Ionic Compounds are listed below:
1) Name the metal (i.e. cation) according to its appearance in the Periodic Table. For example: \[N{a^ + } = Sodium,{\text{ }}M{g^{2 + }} = Magnesium,{\text{ }}A{l^{3 + }} = Aluminum\]
In the present case, \[Mg\] stands for Magnesium.
2) Find the name of the polyatomic ion according to its appearance in the Common Ion Table.
In the present case, \[N{O_3}\] stands for nitrate.
Hence after combining the name of metal and polyatomic ion, the systematic name of \[Mg{\left( {N{O_3}} \right)_2}\] is Magnesium nitrate.
Note: If you are given the systematic name of an ionic compound and you are asked to find the chemical formula of that compound, take into account the charges of each of the elements present in the compound. Make sure that the charges are balanced such that net charge for the compound must be zero.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

The draft of the Preamble of the Indian Constitution class 10 social science CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who gave "Inqilab Zindabad" slogan?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Who is the Brand Ambassador of Incredible India?




