
How would you synthesize alcohol from appropriate alkenes?
Answer
590.1k+ views
Hint: An alcohol is an organic functional group with at least one hydroxyl functional group (-OH) attached to a saturated carbon atom. An alkene is a hydrocarbon that has a double bond between two adjacent carbon atoms.
Complete answer:
-For alkenes to convert to alcohol, there must be the net addition of water across the double bond.
-By hydration of appropriate alkenes, alcohols can be synthesized. The hydrations of alkenes are too slow to behave any significance, hence acid-catalyzed hydration can fasten the synthesis of alcohol.
-The hydration of alkenes can give us an alcohol compound.
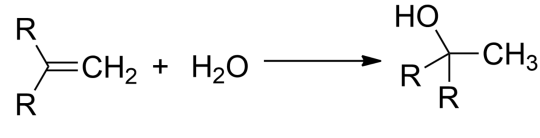
-The breaking of the pi bond in the alkene with an OH bond in water and the formation of a C-H bond and a C-OH bond results in yielding an alcohol product.
-This reaction is an exothermic reaction which yields $10-15kcal/mo{{l}^{-1}}$ energy.
-The mechanism of acid-catalyzed hydration involves the following steps-
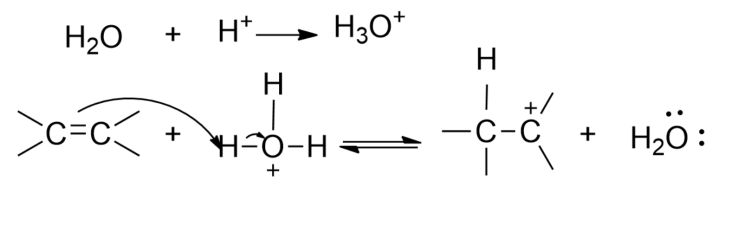
Step1- The electrophilic addition of the proton (or acid) to the double bond forming a carbocation intermediate.
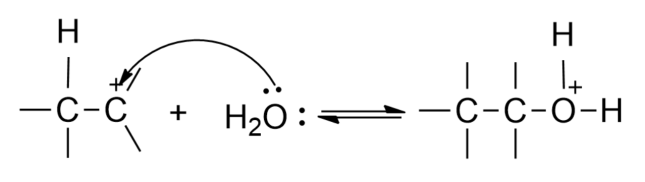
Step 2- - An addition of water afterward which results in the formation of an oxonium ion.
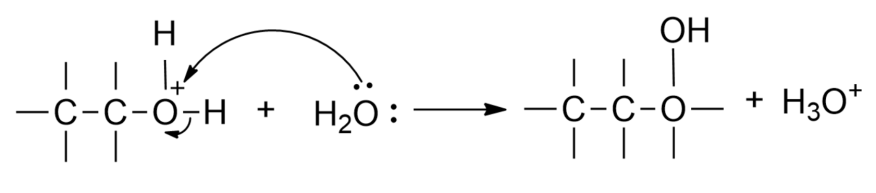
Step 3- Oxonium ion on deprotonation by any base as a catalyst or by another alkene molecule gives the alcohol.
-Another way of synthesis of an alcohol from alkenes can be by oxymercuration or demercuration. According to Markovnikov’s rule, the reaction of alkenes with mercuric acetate followed by the reduction gives alcohol.
$C{{H}_{2}}C{{H}_{2}}\xrightarrow[{{H}_{2}}O/NaB{{H}_{4}}]{(C{{H}_{3}}COO)Hg}C{{H}_{3}}C{{H}_{2}}OH$
-According to anti-Markovnikov’s rule, alkenes on hydroboration-oxidation in the presence of diborane and hydrogen peroxide give alcohol.
\[C{{H}_{3}}CHC{{H}_{2}}\xrightarrow[{{H}_{2}}{{O}_{2}}]{{{B}_{2}}{{H}_{6}}}C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}OH\]
Note:
Protonation of the alkene gives a carbocation. The order of stability of the alkyl cations is
$3{}^\circ >2{}^\circ >1{}^\circ $.Therefore protonation occurring at less substituted carbon will create the more substituted carbocation where the water adds. The addition of a proton at the less substituted carbon and the –OH to the more substituted carbon is known as Markovnikov’s rule.
Complete answer:
-For alkenes to convert to alcohol, there must be the net addition of water across the double bond.
-By hydration of appropriate alkenes, alcohols can be synthesized. The hydrations of alkenes are too slow to behave any significance, hence acid-catalyzed hydration can fasten the synthesis of alcohol.
-The hydration of alkenes can give us an alcohol compound.
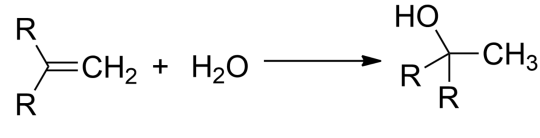
-The breaking of the pi bond in the alkene with an OH bond in water and the formation of a C-H bond and a C-OH bond results in yielding an alcohol product.
-This reaction is an exothermic reaction which yields $10-15kcal/mo{{l}^{-1}}$ energy.
-The mechanism of acid-catalyzed hydration involves the following steps-
Step1- The electrophilic addition of the proton (or acid) to the double bond forming a carbocation intermediate.

Step 2- - An addition of water afterward which results in the formation of an oxonium ion.

Step 3- Oxonium ion on deprotonation by any base as a catalyst or by another alkene molecule gives the alcohol.

-Another way of synthesis of an alcohol from alkenes can be by oxymercuration or demercuration. According to Markovnikov’s rule, the reaction of alkenes with mercuric acetate followed by the reduction gives alcohol.
$C{{H}_{2}}C{{H}_{2}}\xrightarrow[{{H}_{2}}O/NaB{{H}_{4}}]{(C{{H}_{3}}COO)Hg}C{{H}_{3}}C{{H}_{2}}OH$
-According to anti-Markovnikov’s rule, alkenes on hydroboration-oxidation in the presence of diborane and hydrogen peroxide give alcohol.
\[C{{H}_{3}}CHC{{H}_{2}}\xrightarrow[{{H}_{2}}{{O}_{2}}]{{{B}_{2}}{{H}_{6}}}C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}OH\]
Note:
Protonation of the alkene gives a carbocation. The order of stability of the alkyl cations is
$3{}^\circ >2{}^\circ >1{}^\circ $.Therefore protonation occurring at less substituted carbon will create the more substituted carbocation where the water adds. The addition of a proton at the less substituted carbon and the –OH to the more substituted carbon is known as Markovnikov’s rule.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




